Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate whether treatment with foslevodopa/foscarbidopa (LDP/CDP), a continuous (24‑hour/day) subcutaneous infusion of carbidopa/levodopa prodrugs, improves motor fluctuations and quality of life (QoL) in patients at an initial stage of advanced Parkinson’s disease (aPD).
Background: It can be difficult to determine the optimal window to maximize benefit from device-aided therapies. Patients may benefit from initiation earlier in the course of advancing PD. Findings from phase 3 studies evaluating LDP/CDP in patients with aPD have demonstrated clinically meaningful improvement in PD symptoms. There is a lack of data as to whether LDP/CDP treatment is beneficial in patients recently transitioned to aPD.
Method: This Post hoc analyses from a 52-week, phase 3, open-label study (NCT03781167) with LDP/CDP focused on a subgroup of patients aged ≤65 years, with a Hoehn and Yahr stage ≤2 (“On” state), and time since motor fluctuations ≤3 years. The mean change from baseline to final available visit in “Off” time, “On” time without dyskinesia, “On” time without troublesome dyskinesia, and QoL as measured by the 39-Item Parkinson’s Disease Questionnaire (PDQ-39) were assessed. “Off” and “On” times were measured using a 24-hour PD diary and normalized to a 16‑hour waking day. P values presented are nominal; as safety was the primary endpoint, no multiplicity adjustments were made for efficacy endpoints.
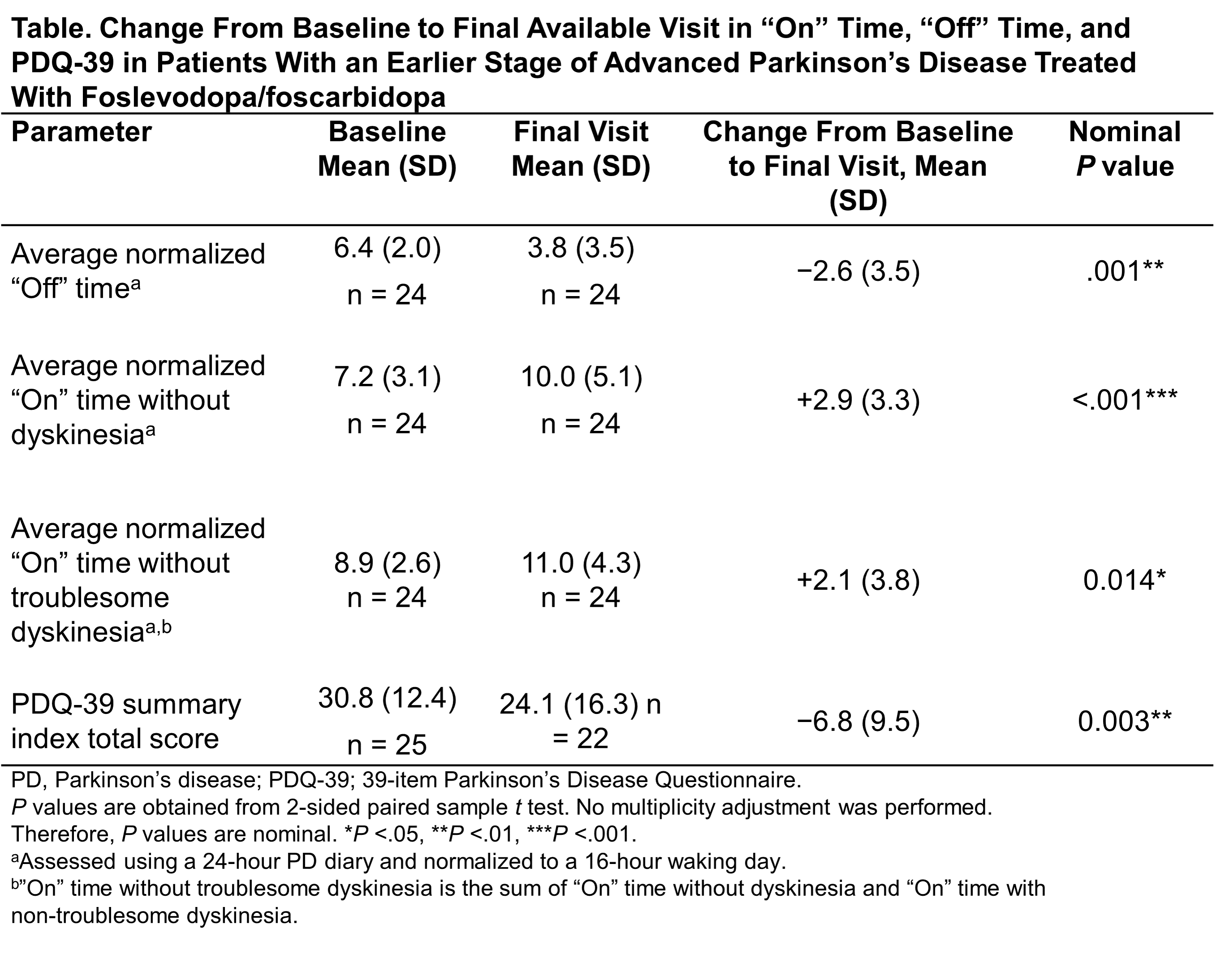
Results: Key baseline characteristics in this subgroup (n=25 of 244 total patients in the study) included a mean (SD) age of 55.8 (8.5) years, and duration of PD <10 years for 84.0% of patients. Treatment with LDP/CDP led to an improvement from baseline to final visit in the mean “Off” time (−2.6 hours, nominal P =.001), average “On” time without dyskinesia (+2.9 hours, nominal P <.001), and average “On” time without troublesome dyskinesia (+2.1 hours, nominal P =.014) [table]. Clinically meaningful improvements were also seen in PDQ-39 scores (mean change from baseline to final visit: −6.8, P =.003). Similar trends were observed in 6 patients with the same criteria treated with LDP/CDP in a 12-week, randomized, phase 3, double-blind study (NCT04380142).
Conclusion: Patients with an earlier stage of aPD showed improvement in motor fluctuations and QoL following treatment with LDP/CDP.
To cite this abstract in AMA style:
A. Antonini, B. Bergmans, D. Kern, P. Odin, L. Bergmann, R. Gupta, J. Homola, M. Shah, J. Zamudio, D. Standaert. Improvement in motor symptoms and quality of life in patients with an earlier stage of advanced Parkinson’s disease treated with foslevodopa/foscarbidopa subcutaneous 24 hour infusion [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/improvement-in-motor-symptoms-and-quality-of-life-in-patients-with-an-earlier-stage-of-advanced-parkinsons-disease-treated-with-foslevodopa-foscarbidopa-subcutaneous-24-hour-infusion/. Accessed January 5, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/improvement-in-motor-symptoms-and-quality-of-life-in-patients-with-an-earlier-stage-of-advanced-parkinsons-disease-treated-with-foslevodopa-foscarbidopa-subcutaneous-24-hour-infusion/