Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate long-term safety/tolerability of foslevodopa/foscarbidopa (LDP/CDP) in patients with advanced Parkinson’s disease (aPD) in a phase 3 open-label extension (OLE) study.
Background: LDP/CDP is a soluble formulation of levodopa (LD)/carbidopa prodrugs administered as 24-hour/day continuous subcutaneous infusion (CSCI). In a 52-week, open-label phase 3 study, LDP/CDP demonstrated a favorable risk/benefit profile in patients with aPD (NCT03781167); those who completed the parent trial were eligible to enroll in an OLE (NCT04379050).
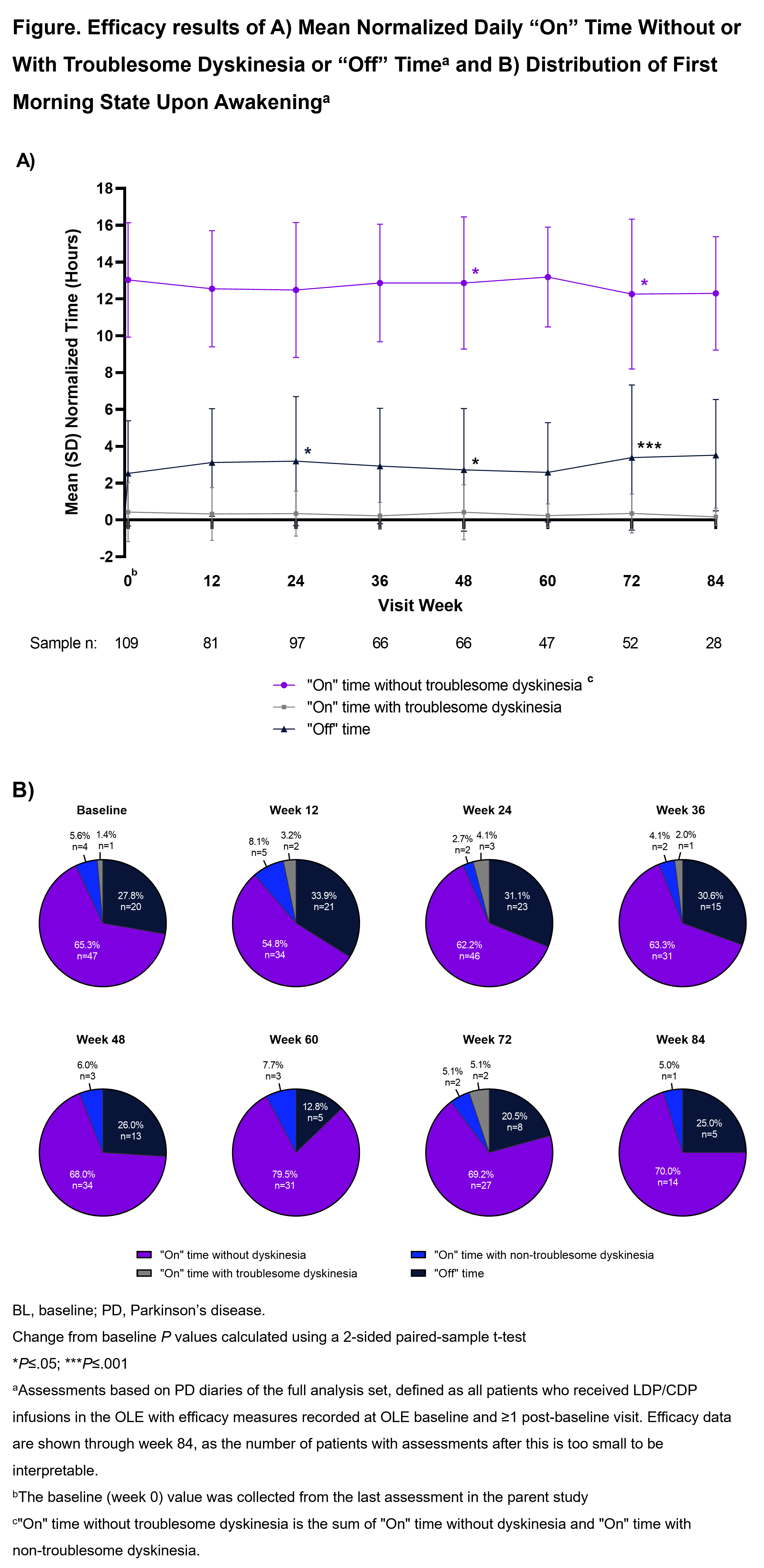
Method: Patients in the parent study were aged ≥30 years with inadequately controlled (≥2.5 hours “Off” time/day), LD-responsive, idiopathic aPD. The ongoing OLE consists of a 96-week Primary Treatment Period and optional Extended Treatment Period. LDP/CDP infusions are individually optimized (600–4250 mg LD equivalents/24 hours). The primary endpoint is safety/tolerability. Key secondary endpoints are change from OLE baseline for mean normalized daily “Off” and “On” times and morning akinesia (“Off” upon awakening). Efficacy data are presented through Week 84 (interim data cutoff August 17, 2022).
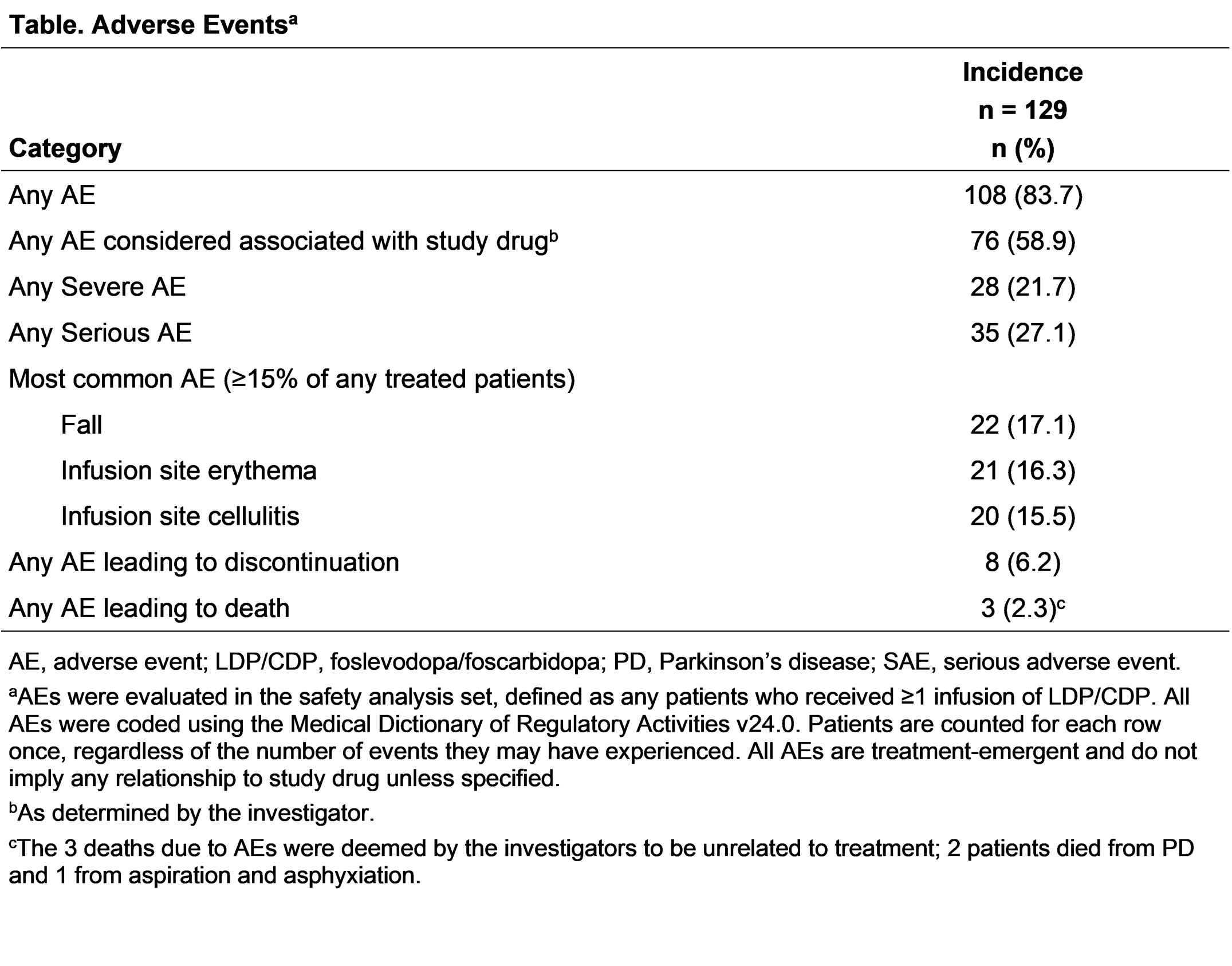
Results: Overall, 137 patients completed the parent study, 129 (94.2%) enrolled in the OLE; 113 (87.6%) are currently ongoing in the OLE. The most common reason for discontinuation was treatment-emergent adverse events (AE; 8 [6.2%]). At OLE baseline, most patients are male (63.6%), White (86.8%), with a mean (SD) age of 63.1 (9.2) years, time since PD diagnosis of 10.2 (4.9) years, and “Off” time of 2.53 (2.9) hours. The mean person-years of study drug exposure (parent study and OLE) is 314.0 years, with a mean (SD) exposure duration of 470.1 (386.9) days. Overall, 83.7% of patients experienced ≥1 AE; most were non-serious and mild or moderate in severity. “Off” and “On” times were generally stable through Week 84 in the OLE (“On” time without troublesome dyskinesia −0.6 [2.9] hours, P=.252); “On” time with troublesome dyskinesia (−0.2 [1.2] hours, P=.364); “Off” time (+0.9 [2.7] hours, P=.102) [figure]. At Week 84, 75% of patients reported awakening in the “On” state (n/N= 15/20).
Conclusion: Long-term CSCI of LDP/CDP was generally safe and well tolerated and effected sustained improvements in motor fluctuations and morning akinesia that were consistent with the parent study.
To cite this abstract in AMA style:
V. Fung, J. Aldred, F. Bergquist, E. Danielsen, A. Jeong, J. Jia, A. Spiegel, S. Talapala, C. Carroll. Open-label Extension Study of Long-term Safety and Tolerability of Foslevodopa/Foscarbidopa for Treatment of Advanced Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/open-label-extension-study-of-long-term-safety-and-tolerability-of-foslevodopa-foscarbidopa-for-treatment-of-advanced-parkinsons-disease/. Accessed April 2, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/open-label-extension-study-of-long-term-safety-and-tolerability-of-foslevodopa-foscarbidopa-for-treatment-of-advanced-parkinsons-disease/