Objective: To evaluate clinical impact of treatment (LCIG) infusion in patients with advanced PD.
Background: Levodopa/carbidopa intestinal gel (LCIG) infusion has demonstrated more stable plasma concentration than oral levodopa in patients with advanced Parkinson’s Disease (PD). Some randomized controlled clinical trials have shown that LCIG infusion effectively reduces daily ¨off¨ time and motor fluctuations in advanced PD. However, external validation of LCIG infusion out of clinical trials is necessary to optimize this therapy implementation in our daily clinical practice.
Method: This is a single-center retrospective cohort study of consecutive patients diagnosed with advanced PD, and treated with LCIG infusion at a Movement Disorders Unit over 13 years. A total of 24 patients were included. Demographic, clinical and treatment variables were registered, and most of them were analyzed before and after LCIG infusion.
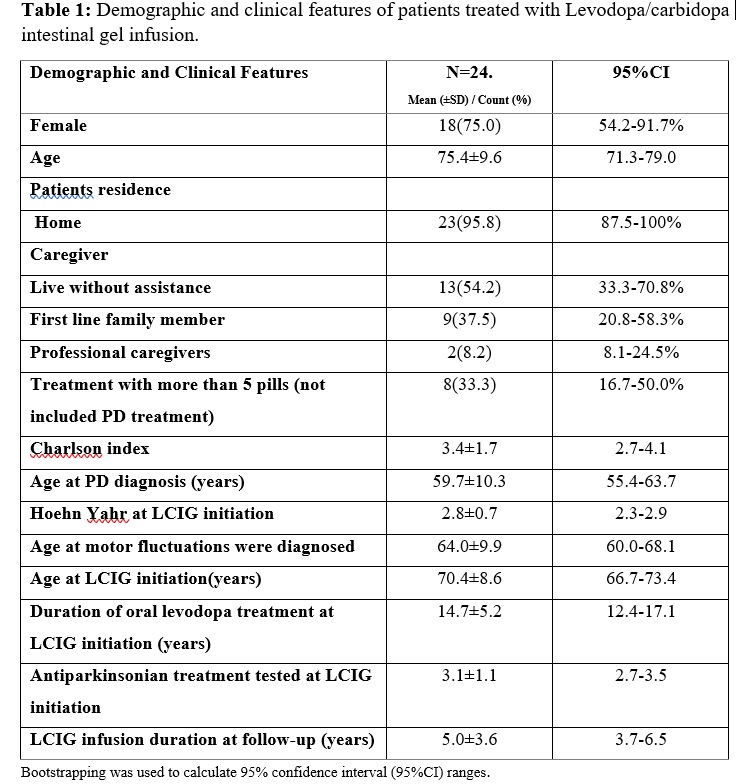
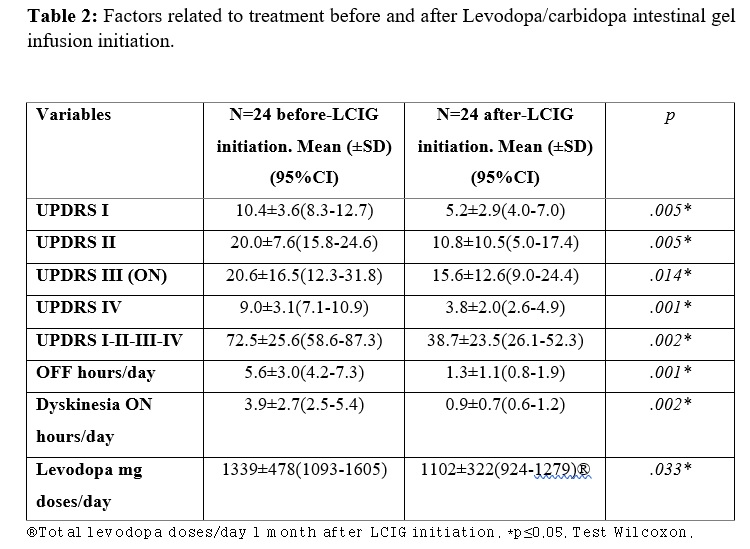
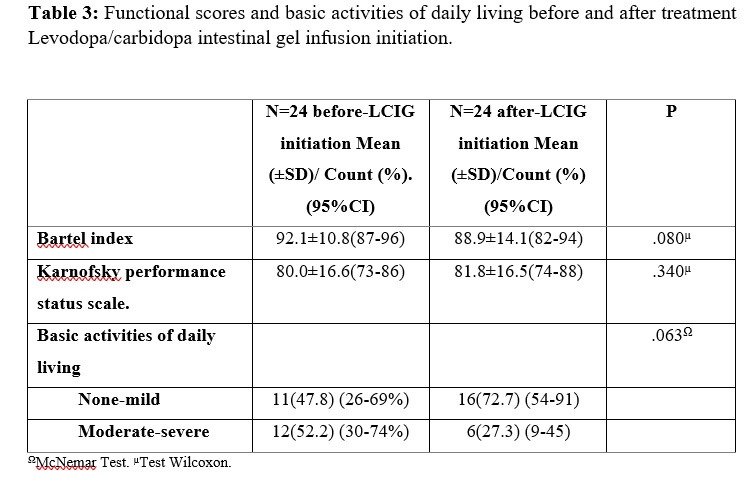
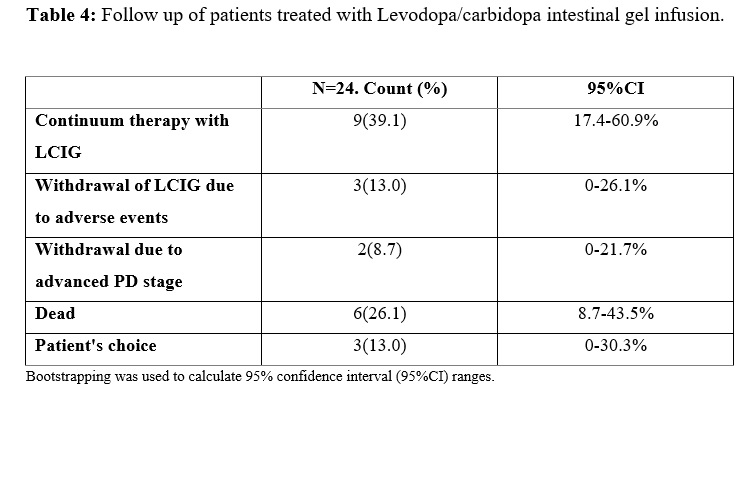
Results: The mean age at PD diagnosis was 59.7±10.3 years, with a female predominance (75%). The mean age that motor fluctuations were diagnosed at was 64.0±9.9, and duration of treatment with oral levodopa until LCIG infusion reached 14.7±5.2 years. This delay from motor fluctuations until LCIG infusion is probably explained by the fact that most patients were treated with apomorphine subcutaneous infusion previous to LCIG. Clinical improvement after LCIG initiation was appreciated according to UPDRS (I-II-III-IV) score (0.002). The OFF hours (5.6±3.0 vs 1.3±1.1, p=0.001), and dyskinesia hours (3.9±2.7 vs 0.9±0.7, p=0.002) per day showed a significant decrease one month after LCIG initiation. The rate of withdrawal from LCIG infusion due to adverse events was low (13%): one due to intestinal perforation, and three patients who presented with severe hallucinations and respiratory failure during SarsCov-2 pandemic, one of whom died because of respiratory complications. 39% of patients maintained a benefit from the therapy.
Conclusion: This real-life experience results are consistent with the data available in the literature. LCIG infusion demonstrated to be a safe and efficacious option for long term patients with advanced PD, and was associated with improvement in UPDRS score (I-II-III-IV), reduction of Off time and ON dyskinesia. The rate of patient withdrawal due to adverse events was relatively low.
To cite this abstract in AMA style:
D. Rivero Rodriguez, G. Tabar Comellas, MI. Morales Casado, AM. Diezma Martin, D. Garcia Melendez, M. Ennazeh Elkhaili, N. López Ariztegui. Clinical analysis of Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease. A single-center retrospective cohort study. [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/clinical-analysis-of-levodopa-carbidopa-intestinal-gel-infusion-in-advanced-parkinsons-disease-a-single-center-retrospective-cohort-study/. Accessed January 4, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinical-analysis-of-levodopa-carbidopa-intestinal-gel-infusion-in-advanced-parkinsons-disease-a-single-center-retrospective-cohort-study/