Category: Parkinsonism, Atypical: PSP, CBD
Objective: To understand the relationship between cholinergic vulnerability and Postural Instability and Gait Disturbances (PIGD) motor features in dementia with Lewy bodies (DLB), and progressive supranuclear palsy (PSP).
Background: Cholinergic vulnerability has been implicated in PIGD motor features in Parkinson’s disease (PD). Atypical parkinsonian syndromes (APS), such as PSP and DLB, may have more severe PIGD motor features than PD. Disease-specific cholinergic system changes in APS may uniquely inform PIGD risk mechanisms.
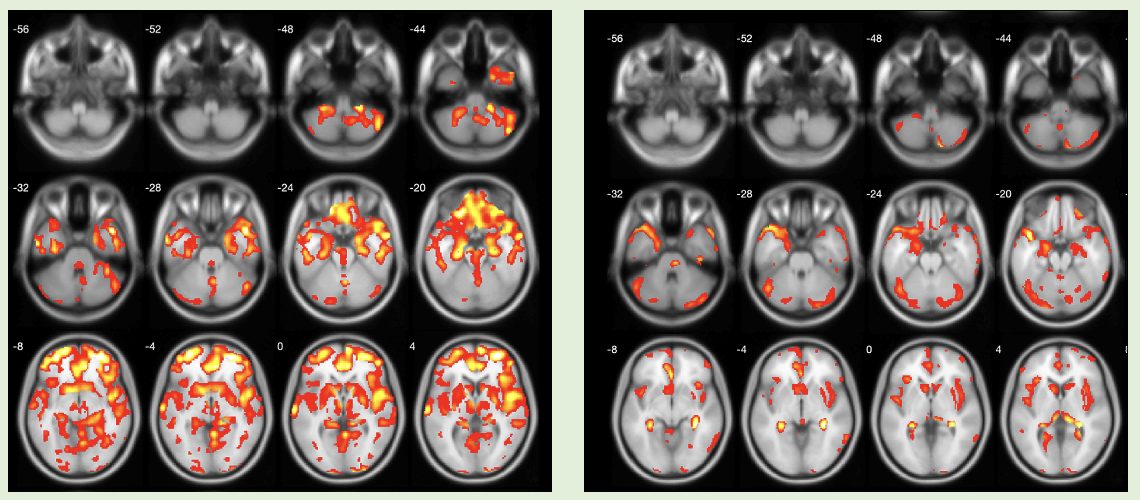
Method: 7 DLB (M6/F1) and 8 PSP (M4/F4) subjects underwent brain VAChT ([18F]-FEOBV) PET and MR imaging. DLB subjects had a mean age of 71.9 (+/-2.9), a mean disease duration of 8.6 years (+/-6.1), and a mean Hoehn & Yahr stage of 3 (+/- 0.5). PSP subjects had a mean age of 70.6 (+/- 4.7), a mean disease duration of 6.7 years (+/- 4.26), and a mean Hoehn & Yahr stage of 4.1 (+/- 0.64). We examined associations between regional VAChT expression and MDS-UPDRS PIGD scores using a statistical nonparametric mapping (SnPM) toolbox.
Results: Whole brain voxel-based correlation with PIGD motor ratings showed lower cholinergic binding in PSP in the cholinergic basal forebrain, septal nucleus, medial temporal lobe, insula, metathalamus, caudate, ant-mid-post cingulum, frontal lobe, cerebellum, and tectum, esp. the superior colliculus (p<0.01). In DLB, correlates of PIGD motor deficits associated with a lower cholinergic binding in right more than left insula, metathalamus, left septal nucleus, right more than left pulvinar, anterior to mid cingulum, pons, and cerebellum (p<0.01).
Conclusion: Although PIGD motor ratings have differential associations with cholinergic vulnerability in APS (e.g. tectum and frontal neocortex in PSP and pons and pulvinar in DLB, overlapping areas were also observed, such as the cholinergic forebrain, limbic (cingulum), paralimbic (insula), and metathalamic regions. Limbic and paralimbic cortices of the brain receive the heaviest cholinergic input from the nucleus basalis of Meynert (Ch4) and are also the principal sources of reciprocal cortical projections back to Ch4. Targeting the cholinergic forebrain may be a novel therapeutic strategy for PIGD motor features not only in DLB but also PSP.
References: Kanel P, Spears CC, Roytman S, Koeppe RA, Frey KA, Scott PJH, et al. Differential cholinergic systems’ changes in progressive supranuclear palsy versus Parkinson’s disease: an exploratory analysis. J Neural Transm (Vienna). 2022;129(12):1469-79. doi: 10.1007/s00702-022-02547-9.
Kanel P, Müller M, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Frey KA, et al. Topography of Cholinergic Changes in Dementia With Lewy Bodies and Key Neural Network Hubs. The Journal of neuropsychiatry and clinical neurosciences. 2020;32(4):370-5. doi: 10.1176/appi.neuropsych.19070165.
To cite this abstract in AMA style:
P. Kanel, T. Brown, S. Roytman, J. Barr, C C. Spears, N. Bohnen. Topography of cholinergic vulnerability correlates of PIGD motor deficits in DLB and PSP: A [18F]-FEOBV PET study [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/topography-of-cholinergic-vulnerability-correlates-of-pigd-motor-deficits-in-dlb-and-psp-a-18f-feobv-pet-study/. Accessed July 15, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/topography-of-cholinergic-vulnerability-correlates-of-pigd-motor-deficits-in-dlb-and-psp-a-18f-feobv-pet-study/