Category: Parkinson’s Disease: Clinical Trials
Objective: We sought to investigate the effects of SCS in PD, using placebo stimulation, compared to either tonic or burst stimulation paradigms.
Background: Gait disturbances are a major contributor to the disability associated with Parkinson’s disease (PD) but the benefits of available treatments are limited. Spinal cord stimulation (SCS) has emerged as a potential therapeutic approach for gait disturbances in PD. Most studies to date have employed tonic paradigms at suprathreshold stimulation for paresthesias, limiting the ability to evaluate independent effects of SCS in PD. Additionally, gaps in knowledge include the magnitude of placebo effects of SCS.
Method: Two PD patients have been implanted with SCS as part of an ongoing clinical trial (NCT03526991). Two parallel octrode leads (Abbott) were percutaneously implanted in the upper thoracic (T2-T4) epidural space. Participants were randomized to 8 weeks of sham, and subsequently, 8 weeks of tonic or burst stimulation. Motor assessments included the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), the 10-meter walk (10MW), the Timed Up and Go (TUG) test and the New Freezing of Gait Questionnaire (NFOGQ). A validated wearable-based gait analyzer system (LEGSys, BioSensics, USA) was used to characterize gait during in-Lab assessments. A validated pendant sensor (PAMSys, BioSensics, USA) was used to remotely monitor ambulation and activity at home.
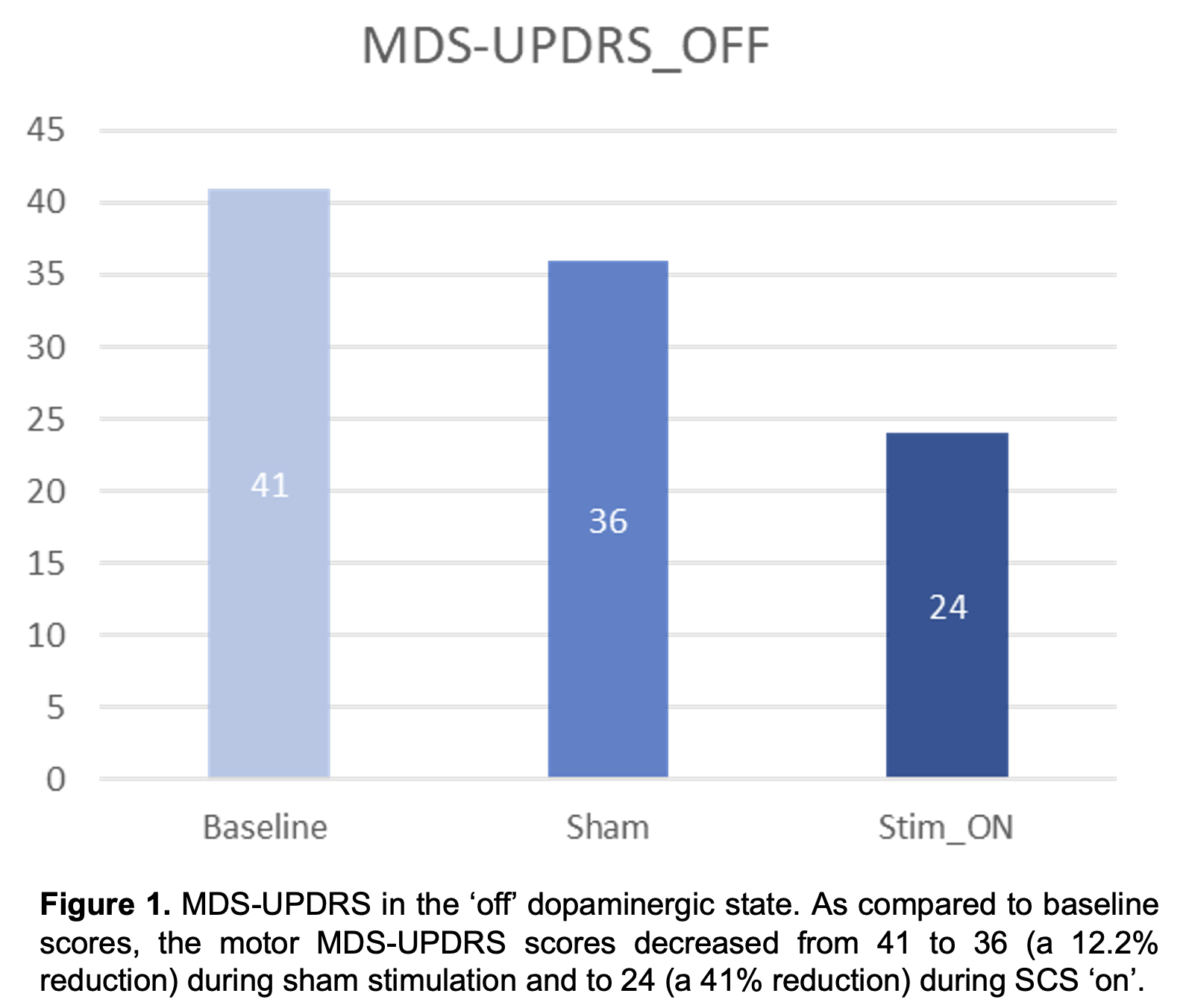
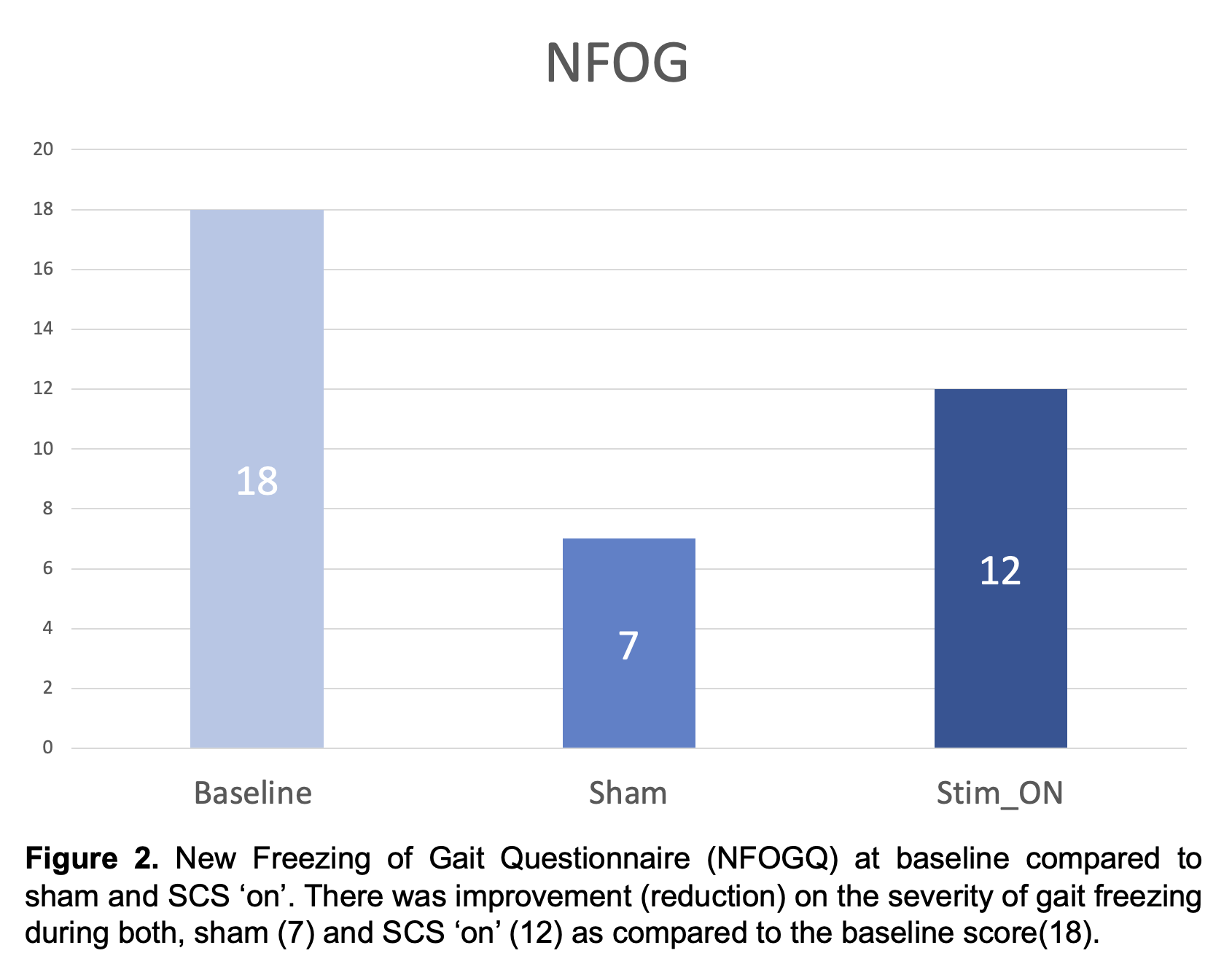
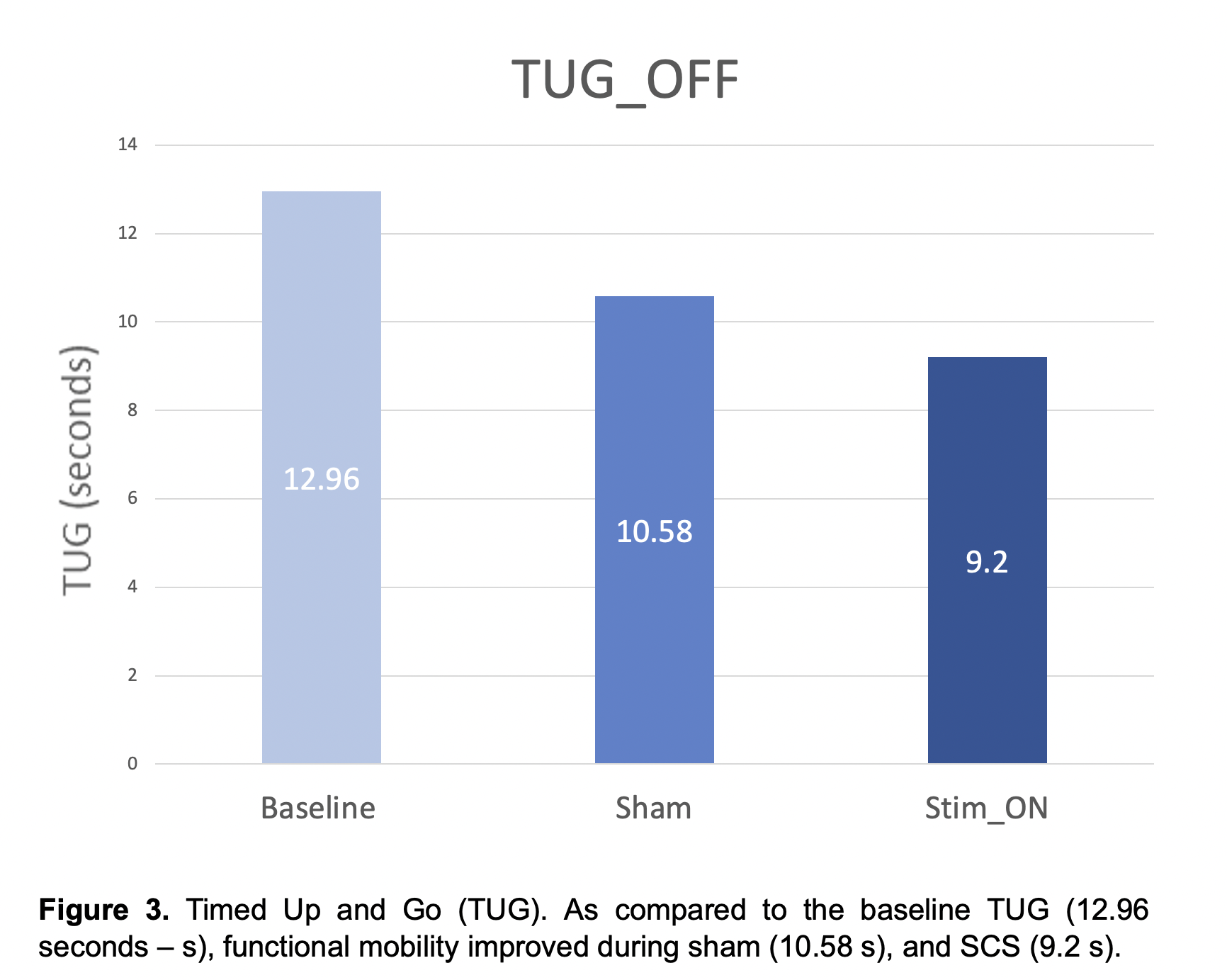
Results: No serious adverse events have been reported in the study participants. Results for the first PD participant who has completed study participation are reported. As compared to baseline, the motor MDS-UPDRS – ‘off’ dopaminergic state – decreased from 41 to 36 (12.2%) during sham and to 24 (41%) during SCS ‘on’ (Fig. 1). Similarly, the NFOGQ improved during both sham (7) and SCS ‘on’ (12) from a baseline score of 18 (Fig 2). Although no changes were observed during the 10MW test, the baseline TUG (12.96 seconds – s) improved during sham (10.58 s), and SCS (9.2 s) (Fig 3).
Conclusion: This is the first randomized, sham controlled study of SCS for PD, comparing two stimulation paradigms. Our preliminary results in one subject, support the safety of SCS in PD and the potential benefits on parkinsonism, gait freezing and functional mobility. Additional, longitudinal evidence on the effects of SCS across PD phenotypes is needed to improve the design of pivotal clinical trials.
To cite this abstract in AMA style:
N. Vanegas-Arroyave, K. Kwei, M. Lee, C. Prakash, L. Branco, A. Tarakad, Y. Taneff, N. Ince, A. Viswanathan, B. Najafi. Exploring the Effects of Spinal Cord Stimulation for Gait Disturbances in Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/exploring-the-effects-of-spinal-cord-stimulation-for-gait-disturbances-in-parkinsons-disease/. Accessed October 16, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/exploring-the-effects-of-spinal-cord-stimulation-for-gait-disturbances-in-parkinsons-disease/