Category: Rating Scales
Objective: This study aimed to explore the possibility of inferring rigidity scores in MDS-UPDRS assessments from other items to enhance the accuracy of clinical evaluations in situations where retrieving all scores may be unfeasible.
Background: Remote assessment of Parkinson’s disease with the MDS-UPDRS scale has been challenging due to the physical handling required for scoring rigidity and postural stability. Previous studies have suggested that rigidity may not be inferable from other items [1,2]. However, theories imply that rigidity and bradykinesia share underlying processes [3,4,5]. In this study, we decided to implement a more advanced methodology to accurately determine the predictability of rigidity scores using other assessment items, in hopes of enhancing the accuracy of clinical evaluations settings where retrieving all scores may be unfeasible.
Method: We used moderate and severe patients [6] from the PPMI dataset [7] and fitted a random forest classifier for each item in each cardinal group. The total predicted score was calculated for each group and compared with the real group score and real total motor score. Inter-rater ICC (calculated with data from [8]) was used as gold standard performance, while a random imputation of scores was used as a control.
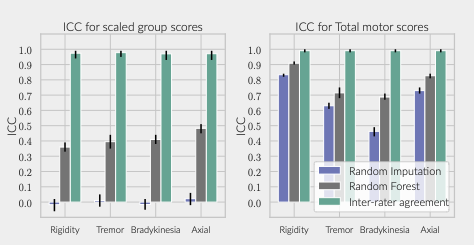
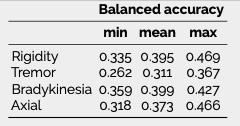
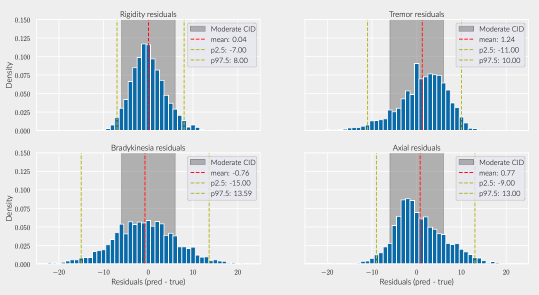
Results: The inferred group score for rigidity performed proportionally worse than other groups, but the total retrieved information was sufficient to achieve an agreement above 90% with the real total motor score [figure 1]. While the balanced accuracy for individual item prediction was low for all groups [table1], 91% of the residuals for rigidity were within the range of a moderate clinically important difference (CID) [figure 2] [9], whereas less than 80% of other groups’ residuals fitted within this range.
Conclusion: Our findings demonstrate that it is possible to recover some of the lost information for all canonical groups, including rigidity, with a performance that exceeds chance. However, rigidity cannot be reliably deduced from other groups’ ratings with enough certainty to avoid the risk of mislabelling a CID. Despite this, in the context of a full motor assessment, rigidity accounts for the least information compared to other groups. No advantage was found for the inference of rigidity or bradykinesia. On the assumption that these share an underlying mechanism, this is a surprising result and may be considered as evidence against this hypothesis.
References: [1] Z. Lin, R. Guo, C. Zhang, D. Li, X. Qian, and B. Sun, “Mds updrs-iii item-based rigidity and postural stability score estimations: A data-driven approach,” Parkinsonism Related Disorders, vol. 94, pp. 13–14, 2021.
[2] S. Luo, C. G. Goetz, D. Choi, S. Aggarwal, T. A. Mestre, and G. T. Stebbins, “Resolving missing data from the movement disorder society unified parkinson’s disease rating scale: Implications for telemedicine,” Movement Disorders, vol. 37, pp. 1749–1755, 8 2022.
[3] A. Berardelli, J. C. Rothwell, P. D. Thompson, M. Hallett, and A. Berardelli, “Pathophysiology of bradykinesia in parkinson’s disease,” Brain, vol. 124, pp. 2131–2146, 2001.
[4] C. Raza, R. Anjum, and N. ul Ain Shakeel, “Parkinson’s disease: Mechanisms, translational models and management strategies,” 6 2019.
[5] M. M. McGregor and A. B. Nelson, “Circuit mechanisms of parkinson’s disease,” 3 2019.
[6] P. Martínez-Martín, C. Rodríguez-Blázquez, M. Alvarez, T. Arakaki, V. C. Arillo, P. Chaná, W. Fernández, N. Garretto, J. C. Martínez-Castrillo, M. Rodríguez-Violante, M. Serrano-Dueñas, D. Ballesteros, J. M. Rojo-Abuin, K. R. Chaudhuri, and M. Merello, “Parkinson’s disease severity levels and mds-unified parkinson’s disease rating scale,” Parkinsonism and Related Disorders, vol. 21, pp. 50–54, 1 2015.
[7] T. P. progression marker initiative (PPMI), “Parkinson’s progression markers initiative.”
[8] K. Sibley, C. Girges, J. Candelario, C. Milabo, M. Salazar, J. O. Esperida, Y. Dushin, P. Limousin, and T. Foltynie, “An evaluation of kelvin, an artificial
intelligence platform, as an objective assessment of the mds updrs part iii,” Journal of Parkinson’s Disease, vol. 12, pp. 2223–2233, 2022.
[9] L. M. Shulman, A. L. Gruber-Baldini, K. E. Anderson, P. S. Fishman, S. G. Reich, and W. J. Weiner, “The clinically important difference on the unified parkinson’s disease rating scale patients: Six hundred fifty-three patients with pd,” Arch Neurol, vol. 67, pp. 64–70, 2010.
To cite this abstract in AMA style:
F. Duque Quiceno, Y. Dushin, G. Sarapata, S. Komarov, A. Matveev, J. O'Keeffe. Inferring Rigidity Scores in MDS-UPDRS Motor Assessment from Other Items [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/inferring-rigidity-scores-in-mds-updrs-motor-assessment-from-other-items/. Accessed April 1, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/inferring-rigidity-scores-in-mds-updrs-motor-assessment-from-other-items/