Objective: To report preliminary results of a 12-week, prospective, multicenter, observational study of the efficacy and safety of LECIG for the treatment of advanced Parkinson´s disease (PD).
Background: Levodopa-entacapone-carbidopa (LECIG) intestinal gel is novel formulation of infusion-based therapy for patients with advanced PD. Initial reports show that LECIG therapy is well tolerated and has good patient-reported efficacy. However, there are currently limited reports of its impact on motor and non-motor PD symptoms and on patients’ quality of life (QoL) in the clinical practice setting.
Method: In this study we evaluated different clinical parameters, safety (adverse events) and levodopa-equivalent daily dose (LEDD) at baseline and after 12 weeks in 9 patients with advanced PD undergoing treatment with LECIG. Clinical measurements included changes in daily motor ‘off’ time, Unified Parkinson Disease Rating Scale (UPDRS) motor scores and Non-Motor Symptoms Scale (NMSS) scores. The Parkinson Disease Questionnaire 8-Item (PDQ-8) was used to assess the impact of LECIG therapy on QoL.
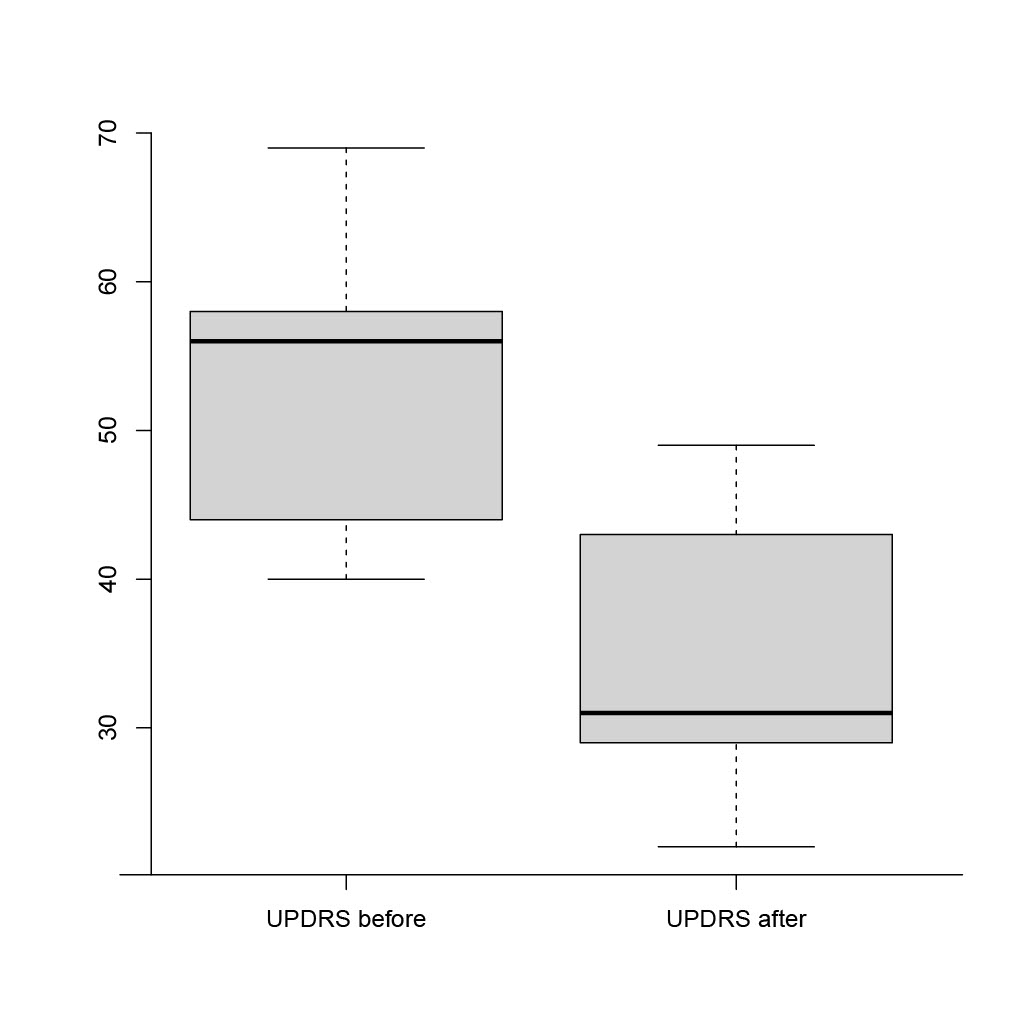
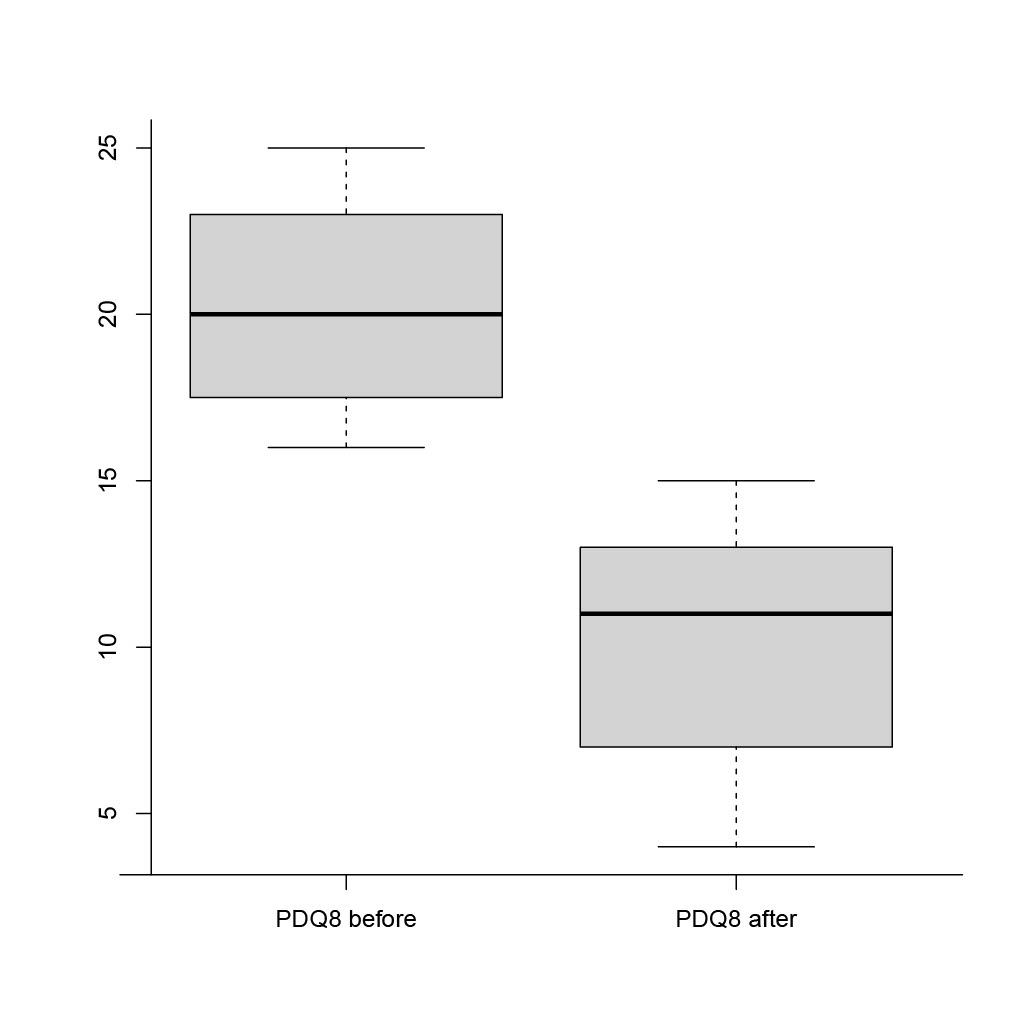
Results: The mean (±SD) age of enrolled patients was 65.3 ± 9.3 years with the mean duration of PD 13 ± 7 years. For all patients LECIG was a de-novo treatment procedure. Baseline clinical assessments were as follows: mean (±SD) UPDRS motor score 52.7 ± 20.3; NMSS score 101.2 ± 20.8; PDQ-8 score 21 ± 4; mean daily ‘off’ time was 5.3 hours. Baseline LEDD was 1,561.7 ± 790.4 mg. After 12 weeks of LECIG therapy, UPDRS, NMSS and PDQ-8 scores, and LEDD, had all significantly decreased versus baseline (p< 0.01; Fig. 1 and 2). Mean daily ‘off’ time decreased by 3.2 hours. One patient with pronounced nocturnal motor symptoms and biphasic dyskinesia was treated with 24-hour LECIG infusion. We observed sustained clinical improvement, including in biphasic dyskinesia (a reduction of 54 in the Unified Dyskinesia Rating Scale total score), in that patient. Adverse events included procedural abdominal pain in 4/9 patients (44.4%) and transient diarrhea in 1/9 patients (11.1%).
Conclusion: The preliminary results of our study show improvements in motor and non-motor PD symptoms and in QoL, as well as good tolerability of LECIG infusion therapy in patients with advanced PD over a period of 12 weeks of treatment.
References: [1 ] Senek M, Nielsen EI, Nyholm D. Levodopa-entacapone-carbidopa intestinal gel in Parkinson´s disease: a randomized crossover study. Mov Disord 2017;32(2):283-286.
[2] Öthman M, Widman E, Nygren I, Nyholm D. Initial experience of the levodopa–entacapone–carbidopa intestinal gel in clinical practice. J Pers Med 2021;11(254):1-10.
To cite this abstract in AMA style:
P. Bago Rožanković, S. Lasić, F. Borovečki, S. Telarović, R. Perković, V. Vuletić, O. Perković, V. Rački, S. Tomić. Initial clinical experience with levodopa-entacapone-carbidopa intestinal gel infusion (LECIG) for the management of advanced Parkinson´s disease in Croatia [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/initial-clinical-experience-with-levodopa-entacapone-carbidopa-intestinal-gel-infusion-lecig-for-the-management-of-advanced-parkinsons-disease-in-croatia/. Accessed April 18, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/initial-clinical-experience-with-levodopa-entacapone-carbidopa-intestinal-gel-infusion-lecig-for-the-management-of-advanced-parkinsons-disease-in-croatia/