Category: Huntington's Disease
Objective: To describe the study protocol for the iMarkHD study.
Background: Huntington’s disease (HD) is still often defined by the onset of motor symptoms, inversely associated with the CAG repeat expansion size in the huntingtin gene. Although the genetic cause is known, much remains unknown about the mechanisms underlying the development of clinical symptoms, disease progression, and the possibility of clinical subtypes in HD. Moreover, there is a lack of reliable and easily accessible biomarkers that are crucial for the development and evaluation of disease-modifying treatments. In the iMarkHD study, we aim to investigate the change in binding profile of four discrete molecular Positron emission tomography (PET) markers, as well as magnetic resonance (MRI) markers, across different disease stages in people with HD compared to healthy controls. This will be measured annually, for up to two years, to determine suitability as markers for HD disease progression and future treatment response in therapeutic trials.
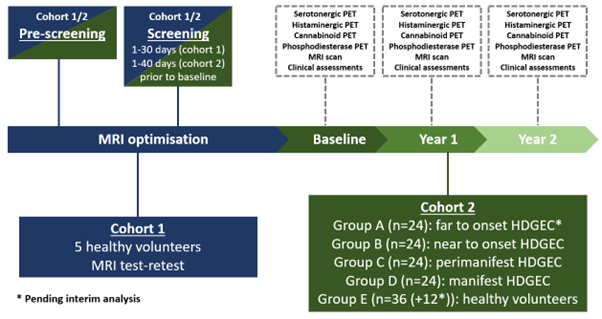
Method: In this single centre neuroimaging study, following MRI optimisation in five healthy volunteers (cohort 1), we aim to recruit 72 people with HD (PwHD) and 36 healthy control participants (cohort 2). Participants in cohort 2 will complete a total of 10 study visits, consisting of a screening visit, followed by a clinical and MRI visit and two PET visits at baseline, year 1, and year 2. PET targets include the cannabinoid 1, histamine 3, and serotonin 2A receptors, as well phosphodiesterase 10A enzyme, whereas MRI outcomes will be multimodal, including but not limited to the assessment of cerebral blood flow, functional connectivity, and brain iron levels.
Results: Cohort 2 of the iMarkHD study started in September 2022 and to date nearly 40 participants have been enrolled. Data collection will be active until at least 2026, and the first interim data analysis results are expected in 2024.
Conclusion: The unique aspect of the iMarkHD study, i.e. its multimodal assessment of possible HD disease markers by combining PET and multi-modal MRI assessments, is likely to enable the provision of a comprehensive examination of the molecular, functional, and structural framework of HD progression in the brain. As such, the iMarkHD study will provide a solid base for the identification of treatment targets and novel outcome measures for future clinical trials.
To cite this abstract in AMA style:
D. van Wamelen, T. Wood, M. Veronese, C. Ginestet, N. Martin, O. Makos, J. Badenoch, M. Hartmann, A. Rangel Cristales, E. Rabiner, R. Gunn, S. Tabrizi, A. Wood, S. Williams. Study protocol for the iMarkHD study in individuals with Huntington’s disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/study-protocol-for-the-imarkhd-study-in-individuals-with-huntingtons-disease/. Accessed December 29, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/study-protocol-for-the-imarkhd-study-in-individuals-with-huntingtons-disease/