Category: Parkinson’s Disease: Clinical Trials
Objective: This exploratory trial aims to compare treatment outcomes of adding one daily dose of opicapone (OPC) 50 mg versus an additional dose of levodopa (L-dopa)/dopa decarboxylase inhibitor (DDCi) in patients with Parkinson’s disease (PD) who develop wearing-off symptoms.
Background: Wearing-off symptoms often develop with long-term treatment with L-dopa in patients with PD. OPC is a once-daily catechol-O-methyltransferase inhibitor with proven efficacy in reducing wearing-off symptoms. The ADOPTION study is designed evaluate the effects of adding OPC compared to an additional dose of L-dopa/DDCi as an approach to treat wearing-off in patients with PD.
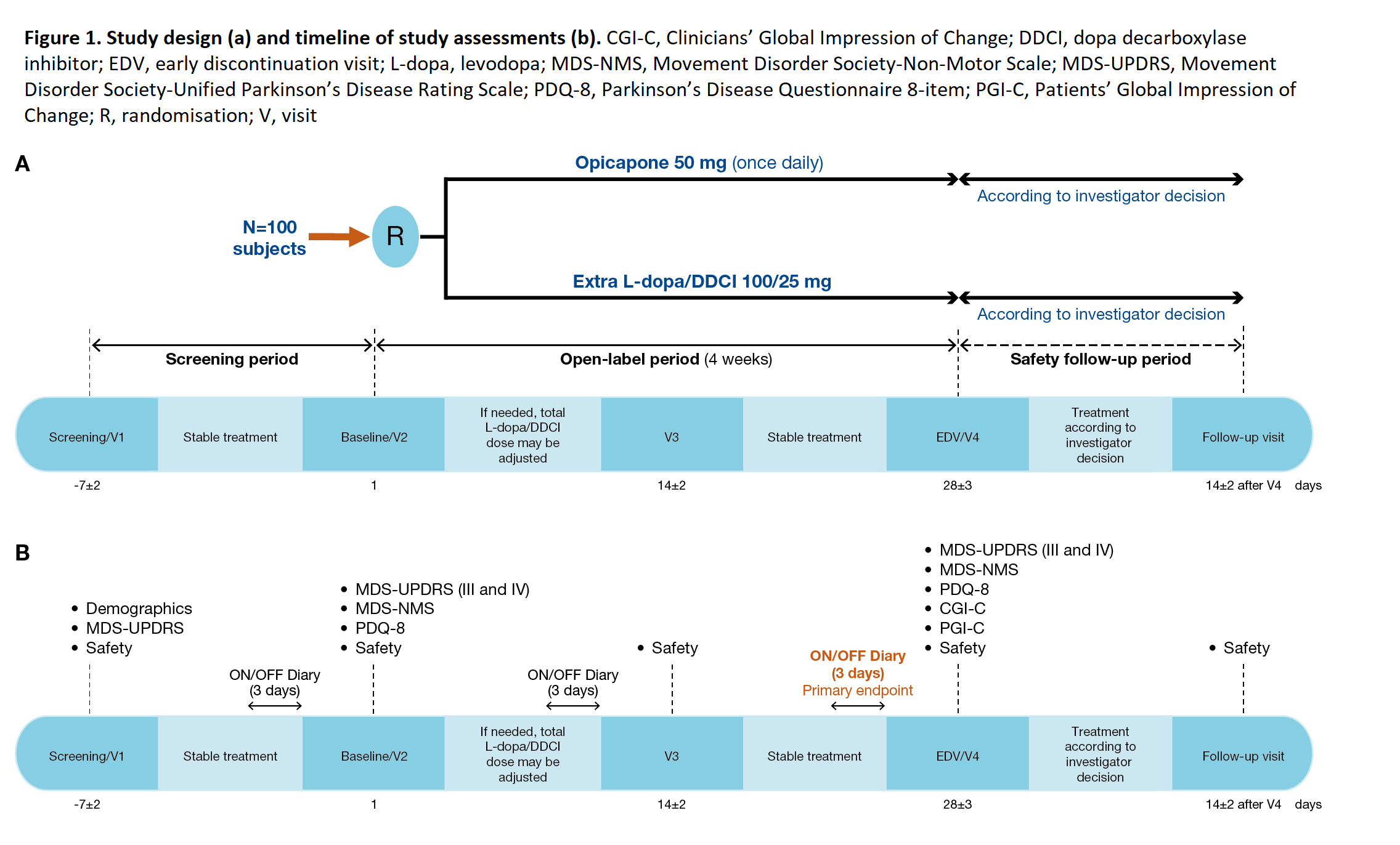
Method: ADOPTION is an open-label, exploratory trial that will recruit patients aged ≥30 years with idiopathic PD. Inclusion criteria included: receiving 3−4 daily oral L-dopa doses (up to 600 mg), showing signs of wearing-off (<2 years) and having total daily OFF time ≤5h. Patients will be equally randomised (1:1) to receive OPC 50 mg once daily or to an additional dose of 100/25 mg L-dopa/DDCI during a 4-week open-label evaluation period [figure1]. Approximately 100 patients from 25 sites in 5 different countries are expected to be recruited.
Results: The primary endpoint is change from baseline in OFF time. Secondary endpoints include tolerability, functional motor and non-motor assessments (Movement Disorder Society (MDS)-Unified Parkinson’s Disease Rating Scale, MDS-Non-Motor Symptoms, Parkinson’s Disease Questionnaire-8), and Clinical and Patient Global Impression of Change. The study has been approved in Portugal, Spain, Italy, Germany and the UK with 25 site-initiation-visits being performed and 23 sites actively recruiting. As of December 2022, 63 patients have been randomised and at least 45 have completed the study.
Conclusion: This study will evaluate the potential of adjunctive OPC versus an additional dose of L-dopa/DDCi as first-line approach to treat wearing-off in patients with PD.
Supported by Bial.
To cite this abstract in AMA style:
J. Ferreira, W. Poewe, O. Rascol, F. Stocchi, A. Antonini, R. Costa, D. Magalhães, J. Rocha, P. Soares-da-Silva. Update on the ADOPTION Study: Randomised, Open-Label Exploratory Trial of Opicapone in Parkinson’s Disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/update-on-the-adoption-study-randomised-open-label-exploratory-trial-of-opicapone-in-parkinsons-disease/. Accessed April 1, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/update-on-the-adoption-study-randomised-open-label-exploratory-trial-of-opicapone-in-parkinsons-disease/