Category: Neuropharmacology
Objective: To identify factors that correlate with efficacy and tolerability of amantadine to predict therapeutic outcomes
Background: Amantadine is the only commercially available medication to alleviate levodopa-related dyskinesias (LRD) in Parkinson disease (PD). Other alternatives are to reduce levodopa and adjunctive medications which can then worsen motor symptoms or advanced therapies such as deep brain stimulation surgery or levodopa infusion which can be cost-prohibitive, patient-selective due to their contraindications. However, the clinical experience of many suggest that the tolerability and efficacy of amantadine are highly variable. At present, there is no data which helps predict which patients may be better responders to amantadine. We aimed to identify demographic, clinical and genetic factors that correlate with efficacy and tolerability of amantadine to predict therapeutic outcomes.
Method: Patients with confirmed diagnosis of idiopathic PD and amantadine exposure (n=53) were identified and classified as responders (group 1), nonresponders (group 2), and unable to tolerate due to side effects (group 3). Demographic and clinical data were collected and a biological sample (blood/saliva) for genetic testing was taken for 28 patients to date. Associations of amantadine response with clinical and demographic factors were assessed using univariate analysis. Polygenic risk scores will be calculated using the latest genome wide association data to correlate with therapeutic response.
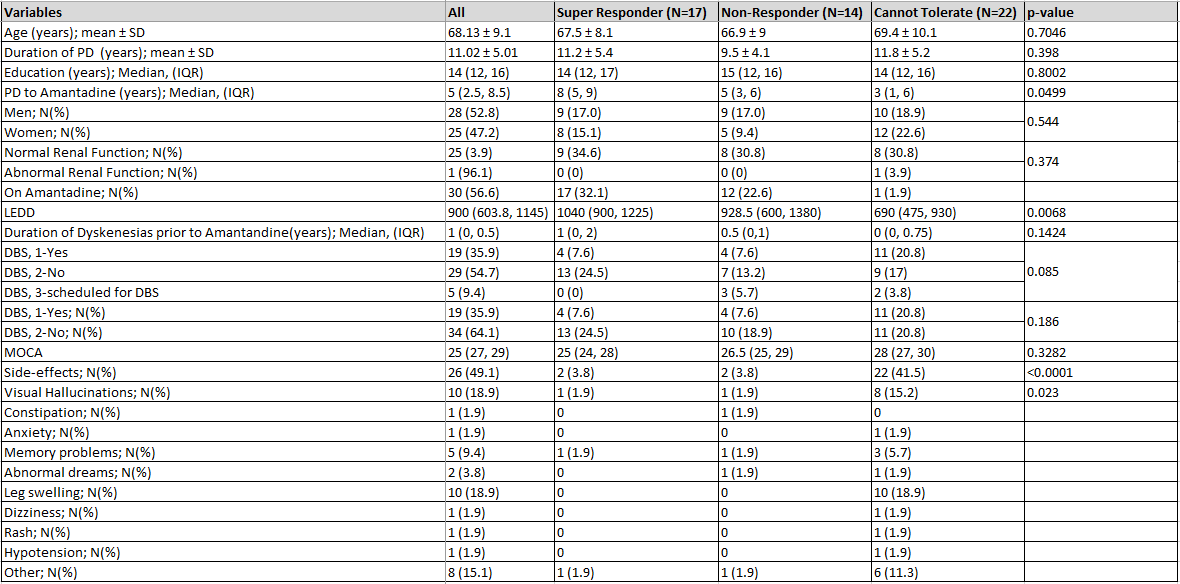
Results: At present, 53 (group 1: n=17, group 2: n=14, group 3: n=22) patients have been identified. [Table 1] The mean age was 68.1 years and 53% (n=28) were male. Mean duration of PD was 11.2, 9.5, and 11.8 years and mean duration of formal PD diagnosis to initiation of amantadine was 8, 5 and 3 years respectively (p =0.049). Median levodopa equivalent daily dose was significantly lower in group 3 (group 1: 1040mg, 2: 928.5mg, 3: 690mg; p =0.007) with 50% (n=11) of group 3 participants having undergone deep brain stimulation. The most common side effect was leg edema (n=10; 18.9%) followed by visual hallucinations (n=8, 16.2%). Genetic analysis is ongoing.
Conclusion: Our cohort underscores the heterogeneity in amantadine response and tolerability. We hope that pharmacogenomic data can shed light into this variability. Results are still being analyzed and will be shared at the Congress.
To cite this abstract in AMA style:
J. Yu, L. Saadatpour, H. Kaur, I. Mata, H. Fernandez. Demographic, clinical and genetic factors affecting amantadine response among patients with Parkinson disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/demographic-clinical-and-genetic-factors-affecting-amantadine-response-among-patients-with-parkinson-disease/. Accessed April 1, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/demographic-clinical-and-genetic-factors-affecting-amantadine-response-among-patients-with-parkinson-disease/