Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate the use of concomitant Parkinson’s disease (PD) medications before and after treatment with levodopa/carbidopa intestinal gel (LCIG) using data from phase 3 clinical trials and real-world studies.
Background: In patients with advanced PD, continuous infusion of LCIG is associated with improvements in PD symptoms and quality of life. LCIG treatment may also reduce the need for concomitant PD medications.
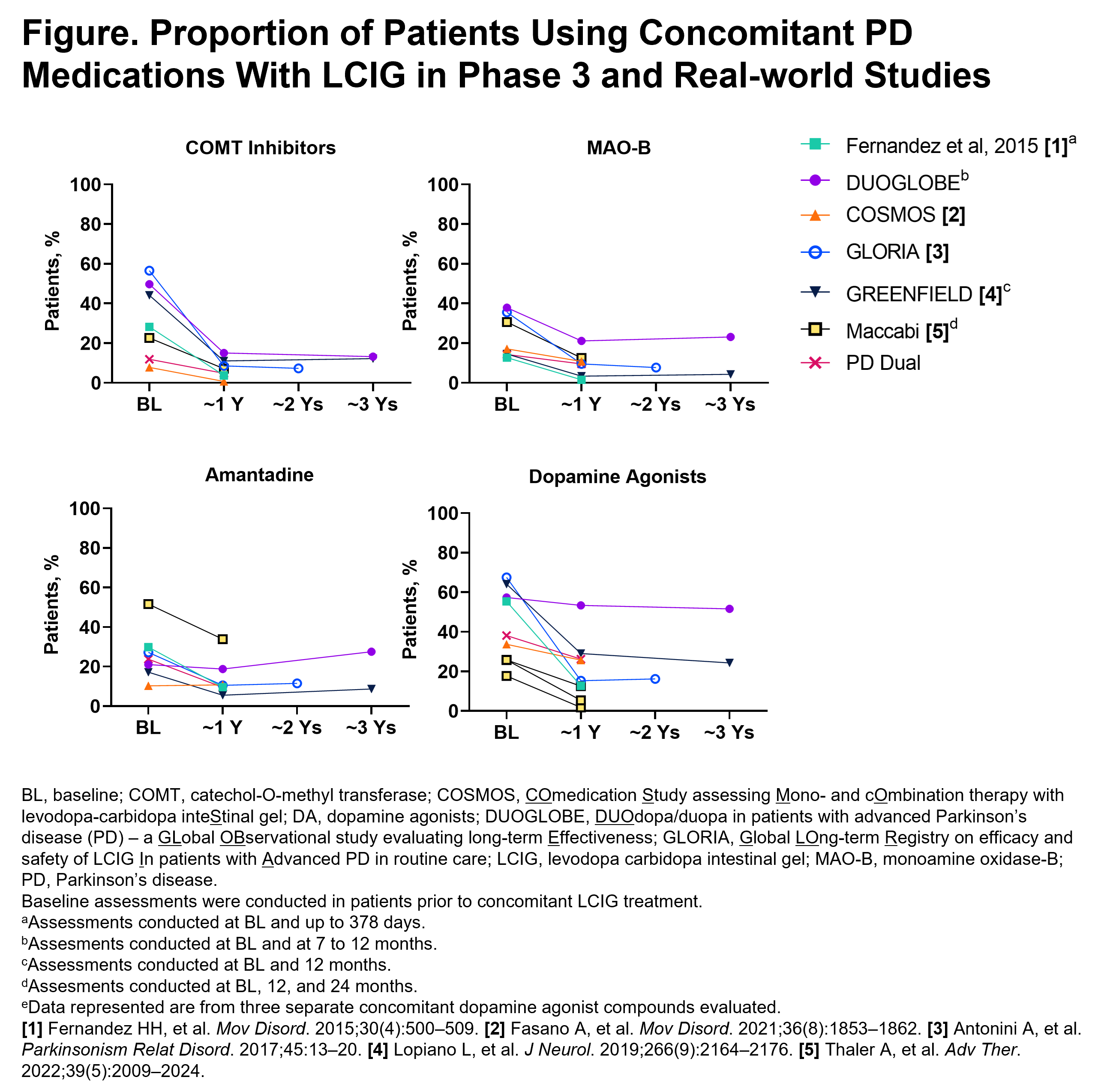
Method: This descriptive analysis reports the percentage of patients using 4 classes of oral/transdermal concomitant PD medications (catechol-O-methyl transferase inhibitors [COMTIs], dopamine agonists [DAs], monoamine oxidase-B inhibitors [MAO-BIs], and amantadine) before initiation of LCIG and after LCIG treatment in 7 phase 3 or real-world studies/analyses. Datasets included a phase 3, open-label trial (NCT00335153; 54 weeks); the COSMOS retrospective observational study (1 year [Y]); the DUOGLOBE prospective, real-world study (3Ys); the GLORIA observational registry (2Ys), the GREENFIELD post-marketing observational study (2Ys); a post-hoc analysis from the Israeli Maccabi health insurance database (reported here over 1Y); and the PD-Dual retrospective chart review (1Y).
Results: A total of 1582 patients with advanced PD who received LCIG treatment in the 7 datasets were included in this analysis. At 1Y, 14.3–38.8% of patients were not using any concomitant PD medications. In general, the proportion of patients using concomitant medication was reduced at approximately 1Y of treatment for all drugs (COMTI: baseline [BL], 7.8–56.5%, 1Y, 0.7–15.0%; MAO-BI: BL, 12.7–37.9%, 1Y, 1.5–21.1%; DA: BL 17.7–67.5%, 1Y, 1.8–53.4%; amantadine: BL,10.3–51.6%, 1Y, 5.5–33.9%), except in DUOGLOBE where DA and amantadine use remained stable. The greatest decrease at 1Y were among COMTIs [figure1]. The use of most concomitant PD medications remained generally stable from 1Y to the last patient visit time point (up to 3Ys) (COMTI, 12.2–13.2%; MAO-BI, 4.3–23.1%; DA, 24.3–51.6%; amantadine, 8.7–27.5%).
Conclusion: Overall, in patients with advanced PD, treatment with LCIG was associated with decreased use of most major classes of oral PD medications, with a considerable proportion of patients using LCIG monotherapy, thereby potentially minimizing the complexity of therapeutic regimens and contributing to reduced pill burden.
References: [1] Fernandez HH, et al. Mov Disord. 2015;30(4):500–509.
[2] Fasano A, et al. Mov Disord. 2021;36(8):1853–1862.
[3] Antonini A, et al. Parkinsonism Relat Disord. 2017;45:13–20.
[4] Lopiano L, et al. J Neurol. 2019;266(9):2164–2176.
[5] Thaler A, et al. Adv Ther. 2022;39(5):2009–2024.
To cite this abstract in AMA style:
J. Boyd, H. Fernandez, W. Poewe, A. Fasano, N. Kovács, K. Chaudhuri, M. Simu, J. Aldred, L. Lopiano, S. Parashos, J. Parra, L. Bergmann, P. Kukreja, M. Shah, O. Ladhani, G. Melzi, C. Yan, A. Antonini. Use of concomitant medications before and after treatment with levodopa/carbidopa intestinal gel in patients with advanced Parkinson’s disease: summary of phase 3 and real-world studies [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/use-of-concomitant-medications-before-and-after-treatment-with-levodopa-carbidopa-intestinal-gel-in-patients-with-advanced-parkinsons-disease-summary-of-phase-3-and-real-world-studies/. Accessed December 16, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/use-of-concomitant-medications-before-and-after-treatment-with-levodopa-carbidopa-intestinal-gel-in-patients-with-advanced-parkinsons-disease-summary-of-phase-3-and-real-world-studies/