Category: Parkinson’s Disease: Clinical Trials
Objective: To identify people at risk of Parkinson’s disease (PD) using a staged-screening paradigm.
Background: Early recognition of people with PD may be essential to the efficacy of disease-modifying therapies. Because PD is not common, and specific tests are costly, a scalable screening process is needed to identify persons at high risk of developing PD clinical features.
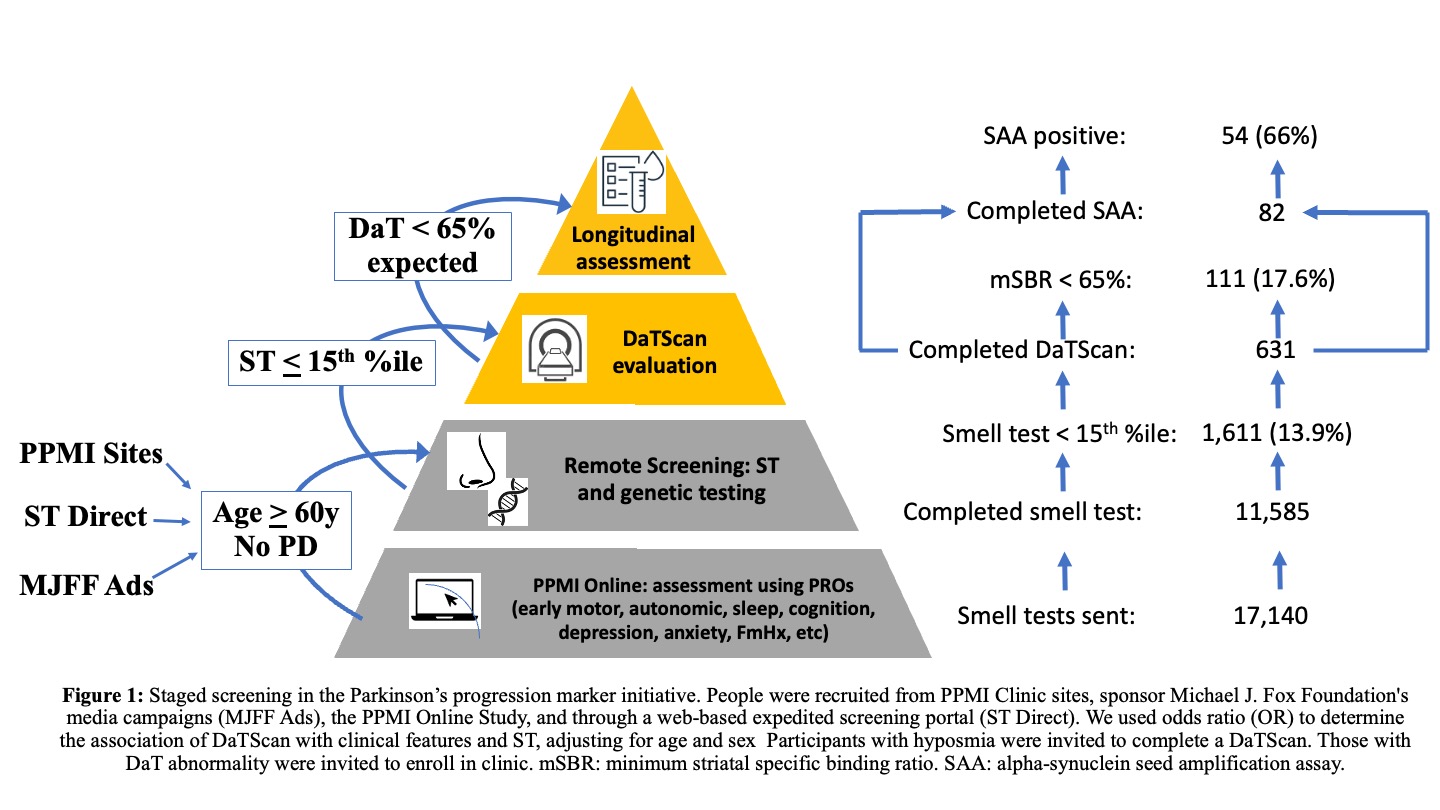
Method: The Parkinson Progression Markers Initiative (PPMI) is an observational cohort study aiming to identify biomarkers in PD. PPMI expanded enrollment of all cohorts with increased focus on identifying persons who may develop PD clinical features. Individuals meeting specific eligibility criteria proceed to additional screening, including smell test (ST) and DaTScan [Figure 1]. Selected participants enroll in longitudinal clinical follow-up with in-person battery of clinical and biomarkers, including cerebrospinal fluid (CSF) alpha-synuclein seed amplification assay (SAA). People were recruited from PPMI Clinic sites, sponsor Michael J. Fox Foundation’s media campaigns (MJFF Ads), the PPMI Online Study, and through a web-based expedited screening portal (ST Direct). We used odds ratio (OR) to determine the association of DaTScan with clinical features and ST, adjusting for age and sex.
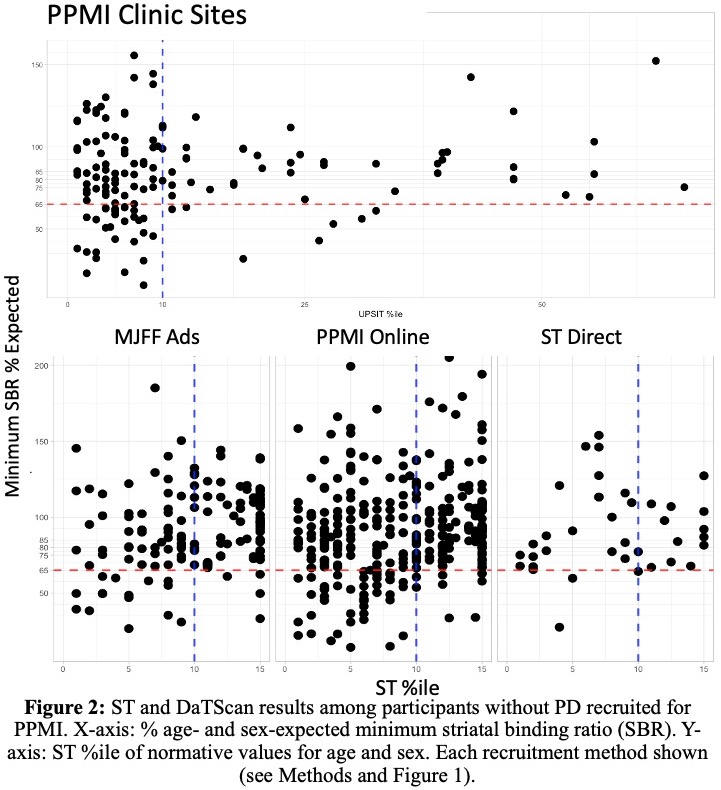
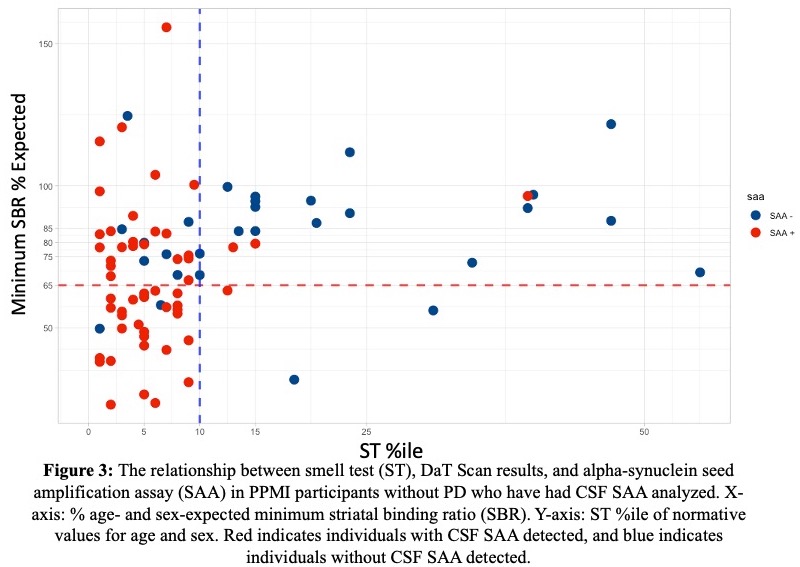
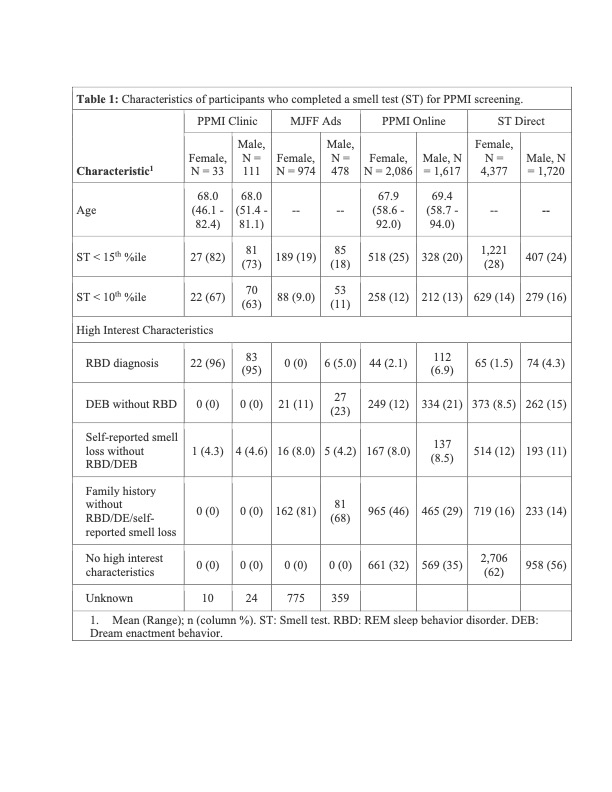
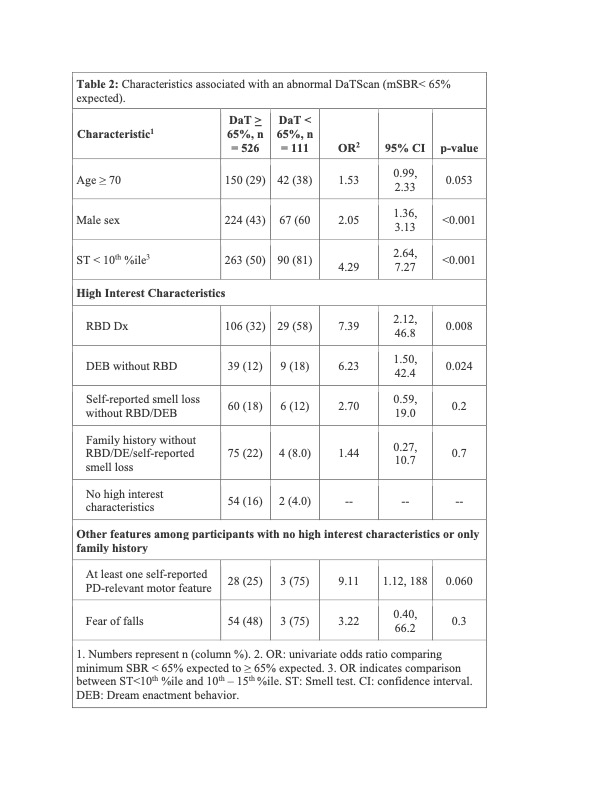
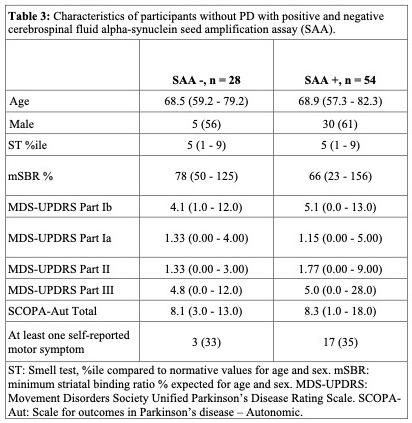
Results: As of February 3, 2023, 11,585 participants had completed a smell test [Table 1]. DaTScan was completed in 631; 111 (17.5%) had an abnormal DaT (minimum striatal specific binding ratio [mSBR] <65% expected for age and sex), indicating a high risk for PD [Figure 2]. ST<10th%ile compared to the 10th–15th%ile was associated with mSBR<65% (aOR: 4.36 [95% CI 2.53, 7.97], p<0.001)[Table 2]. Self-report of REM sleep behavior disorder (RBD) or only dream enactment behavior (DEB) was associated with an mSBR<65% (aOR: 6.94 [2.01, 43.8], p=0.010). Among those without RBD/DEB or self-reported smell loss, report of a PD-relevant motor symptom was associated with mSBR<65% [Table 2]. Among 82 participants with CSF analysis, 54 (65%) were positive for SAA. Over 90% (n=49) had an ST<10th%ile, and half (n=28) had an mSBR<65% [Figure 3]. Clinical features were similar between people with and without SAA [Table 3].
Conclusion: A staged-screening paradigm identifies a biomarker-defined cohort at risk for development of PD clinical features. Longitudinal follow-up will improve understanding of the risk and timeline to PD development.
To cite this abstract in AMA style:
E. Brown, A. Siderowf, T. Simuni, T. Sherer, M. Brumm, C. Caspell-Garcia, R. Kurth, C. Gochanour, M. Kuhl, M. Korell, J. Valverde Twiggs, C. Stanley, L. Concha, C. Soto, K. Marek, C. Tanner. A staged screening paradigm to identify people at risk of Parkinson’s disease in the Parkinson’s Progression Markers Initiative [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/a-staged-screening-paradigm-to-identify-people-at-risk-of-parkinsons-disease-in-the-parkinsons-progression-markers-initiative/. Accessed December 25, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-staged-screening-paradigm-to-identify-people-at-risk-of-parkinsons-disease-in-the-parkinsons-progression-markers-initiative/