Category: Rating Scales
Objective: To assess TDIS and global AIMS measures change trajectories using data from KINECT-4, an open-label 52-week trial assessing valbenazine in TD [1]
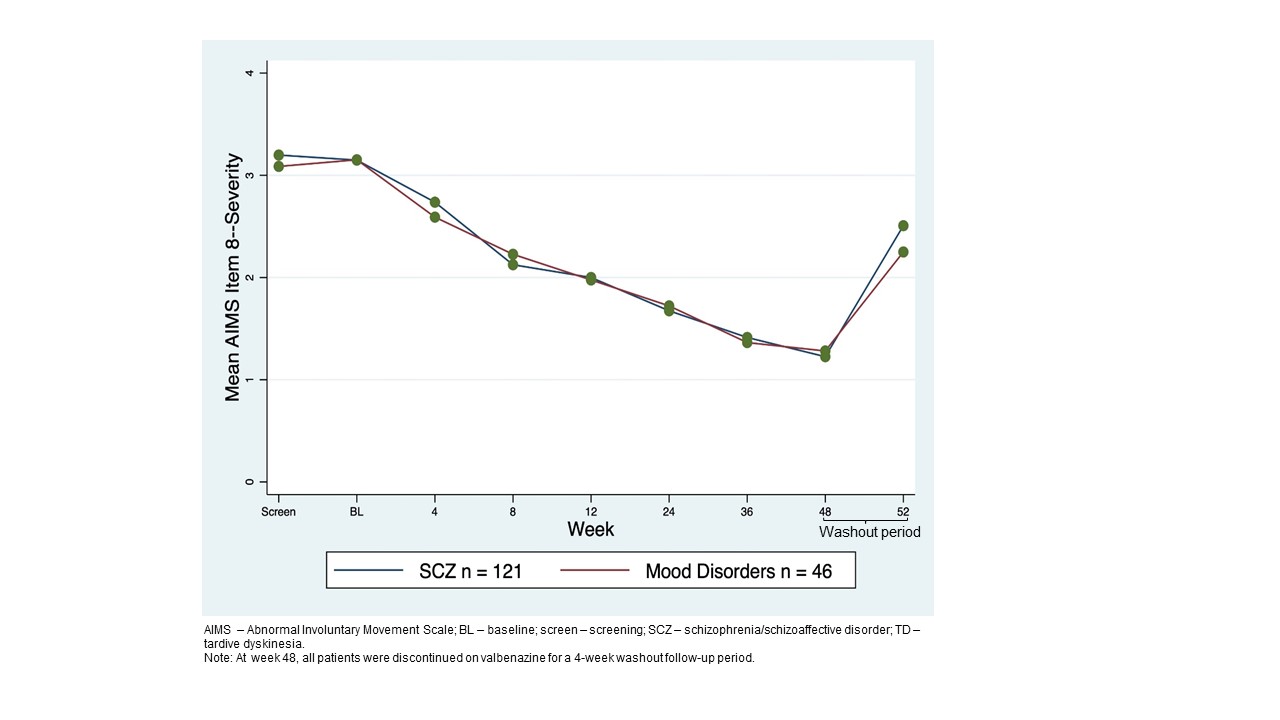
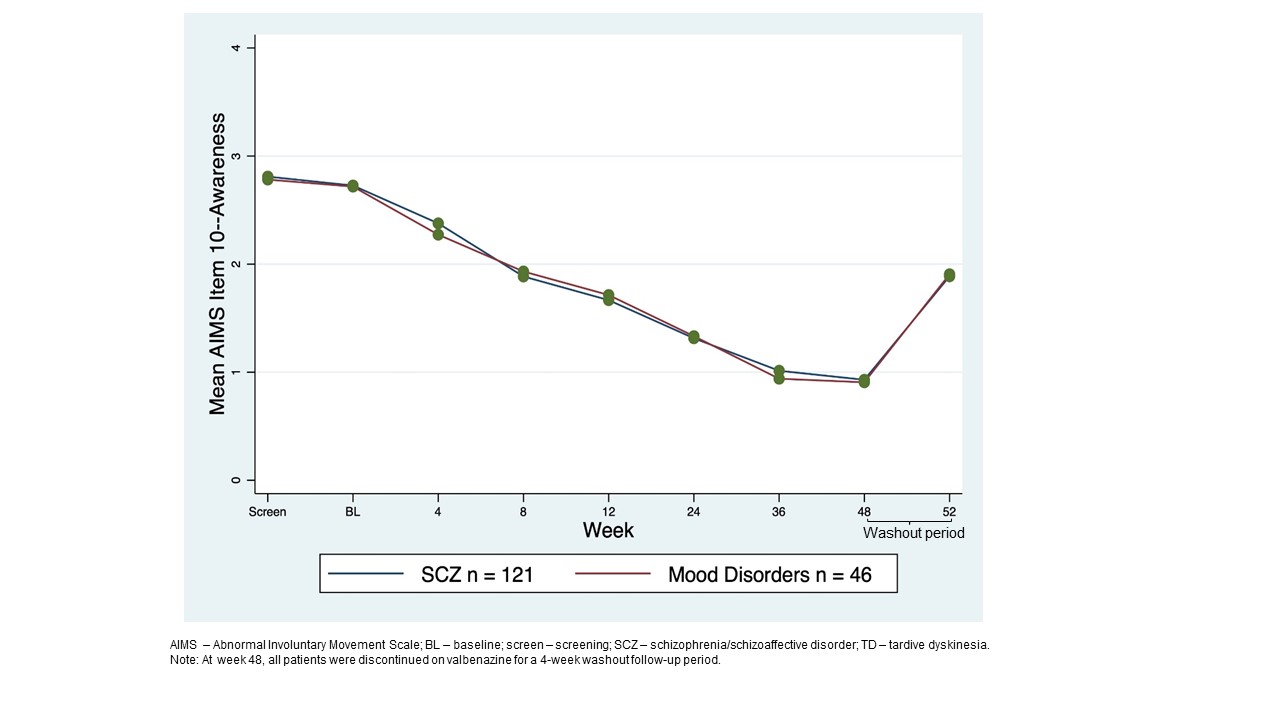
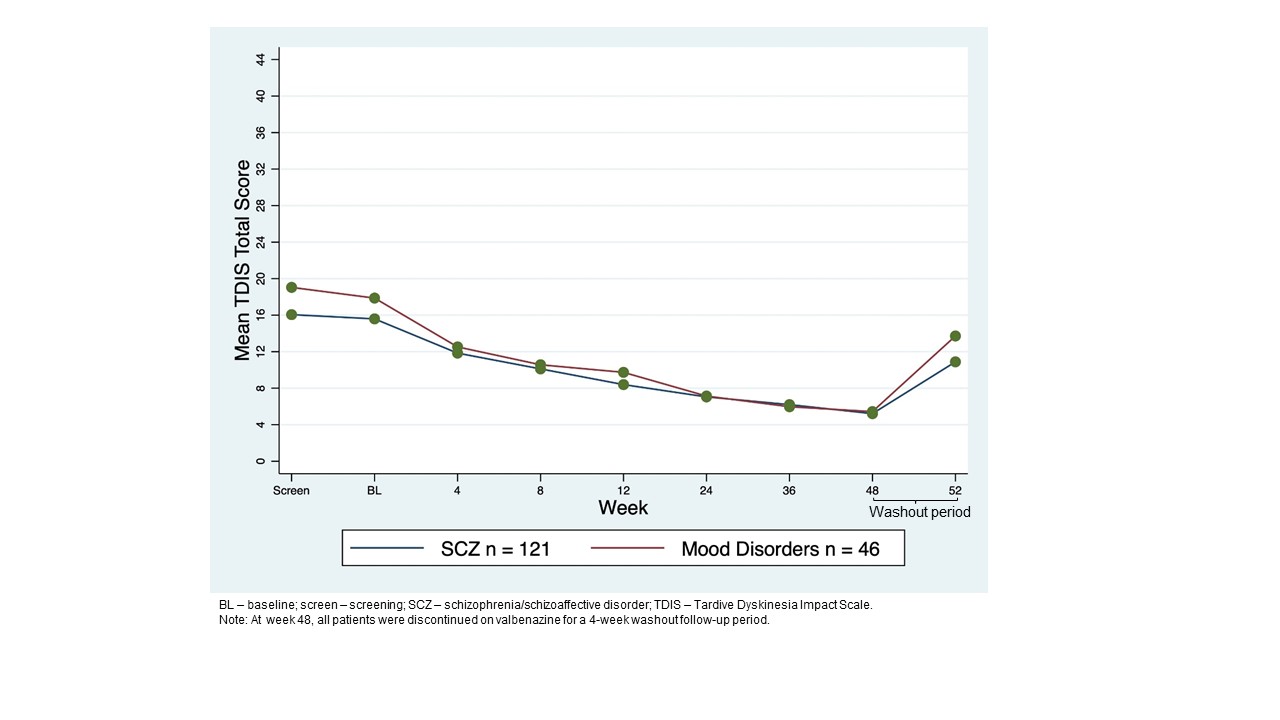
Background: AIMS is a clinician-rated-outcome that longitudinally evaluates signs of TD severity; TDIS is a TD-specific, patient-reported-outcome that assesses physical/social/emotional TD impacts.[2] AIMS uses a 12-item-scale for involuntary movements, including 3 items to measure global judgement. AIMS item 8 (AIMS8) assesses patient’s overall TD severity and AIMS item 10 (AIMS10) assesses TD awareness/distress. Higher scores indicate greater severity and awareness/distress (score: 0-4). TDIS uses a 11-item-scale, and a higher score indicates greater impairment/disability (total score range: 0-44).
Method: AIMS total score (items 1-7), AIMS8, AIMS10, and TDIS total score were analyzed at baseline (BL) through week 52 in the KINECT-4 population and by primary psychiatric diagnosis (schizophrenia/schizoaffective disorder [SCZ] or mood disorder). AIMS was a primary endpoint. TDIS was an exploratory endpoint.
Results: 163 patients who completed 48-week treatment period+4-week washout were analyzed (SCZ n=119; mood disorder n=44). At BL, mean AIMS total score was 14.6. TD severity was moderate (AIMS8 mean=3.2).Most patients were aware of TD with moderate distress (AIMS10 mean=2.7). AIMS total score decreased over 48 weeks, with a mean change from BL of −10.6.[1] TD severity and distress decreased, with most patients scored with minimal severity (AIMS8=1)[figure1] and as aware/no distress at 48 weeks (AIMS10=1)[figure2]. Mean TDIS scores decreased from 16.5 at BL to 6.0 at week 48, indicating TD had little impact on activity by the end of the treatment period which was seen for SCZ and mood disorder groups [figure3]. A weak significant correlation was seen between total AIMS and TDIS scores (Pearson r=0.26).
Conclusion: Though AIMS and TDIS measure unique aspects of TD, TDIS demonstrated a comparable pattern of improvement to AIMS total score, TD severity (AIMS8), and awareness/distress (AIMS10) over 48 weeks across diagnostic groups. This suggests that on average, clinician-rated TD severity and patient-reported TD impact decrease with treatment. As such, use of AIMS and TDIS in future studies may give a more complementary understanding of the patient’s TD experience.
References: 1. Marder SR, Singer C, Lindenmayer JP, et al. A phase 3, 1-year, open-label trial of valbenazine in adults with tardive dyskinesia. J Clin Psychopharmacol. 2019;39(6):620-627.
2. Stull DE, Bron M, Bean S, et al. Impacts of tardive dyskinesia (TD) symptoms on patients: analysis of a TD-specific patient-reported outcome [abstract]. Mov Disord. 2021; 36 (suppl 1). Accessed February 15, 2023.https://www.mdsabstracts.org/abstract/impacts-of-tardive-dyskinesia-td-symptoms-on-patients-analysis-of-a-td-specific-patient-reported-outcome/
To cite this abstract in AMA style:
M. Perez-Rodriguez, D. Stull, R. Dhanda, M. Bron, E. Dunayevich, C. Correll. Trajectories of Tardive Dyskinesia Impact Scale (TDIS) and Abnormal Involuntary Movement Scale (AIMS) over time with valbenazine treatment [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/trajectories-of-tardive-dyskinesia-impact-scale-tdis-and-abnormal-involuntary-movement-scale-aims-over-time-with-valbenazine-treatment/. Accessed January 1, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/trajectories-of-tardive-dyskinesia-impact-scale-tdis-and-abnormal-involuntary-movement-scale-aims-over-time-with-valbenazine-treatment/