Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate the frequency of adverse events over time with foslevodopa/foscarbidopa (LDP/CDP) in patients with advanced Parkinson’s disease (PD) in a phase 3, open-label safety trial over 52 weeks.
Background: LDP/CDP is a highly soluble formulation of levodopa/carbidopa prodrugs delivered as a continuous (24-hour/day) subcutaneous infusion. Data from a 12-week, double-blind, double-dummy study suggest that safety events occur more frequently in the first few weeks after LDP/CDP initiation compared to the subsequent maintenance period.
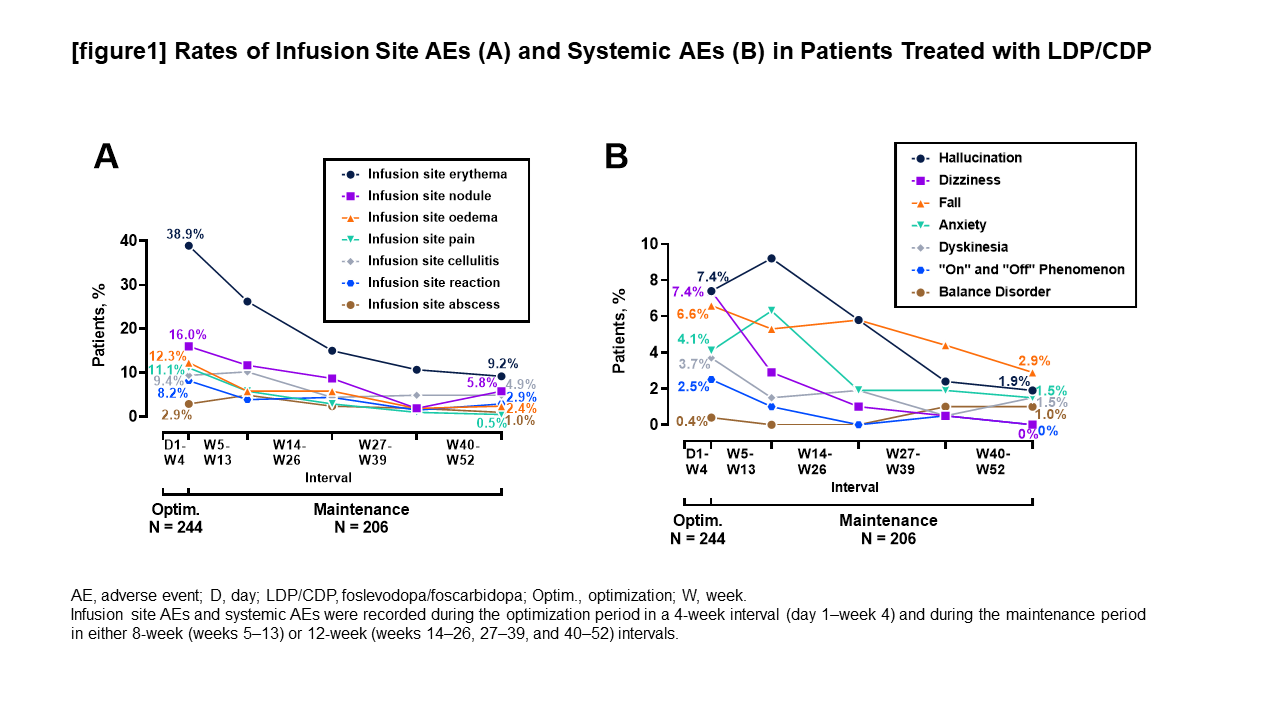
Method: We conducted a post hoc analysis of third interim results from a 52-week, phase 3, open-label, single-arm trial (NCT03781167) of LDP/CDP in levodopa-responsive idiopathic PD patients aged ≥30 years whose symptoms were inadequately controlled by their current therapy (≥2.5 average “Off” hours/day). Individualized doses of LDP/CDP were titrated to optimally control patient motor symptoms during a 4-week optimization period, and optimized doses were maintained for the remainder of the treatment period (48-week maintenance period). Adverse events (AEs) were recorded during the 4-week optimization period in daily/weekly intervals and during the 48-week maintenance period in either 8-week (weeks 5–13) or 12-week (weeks 14–26, 27–39, and 40–52) intervals. AEs were classified as systemic or infusion site AEs.
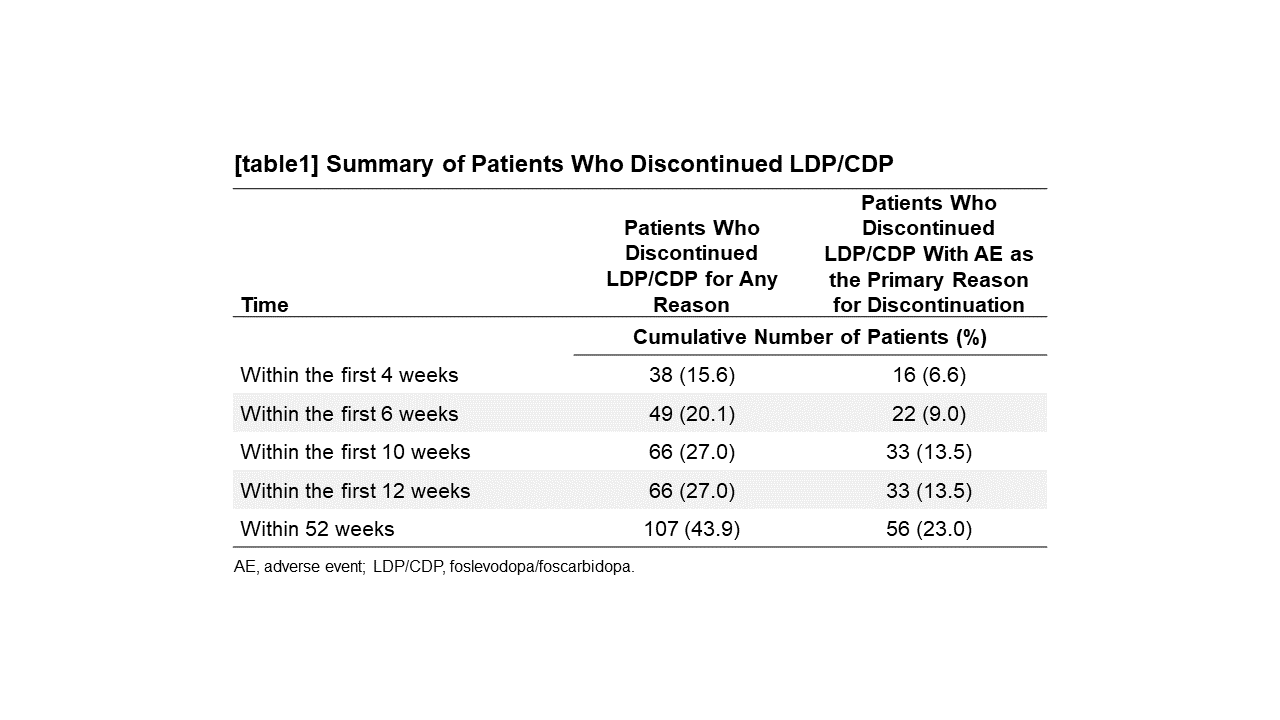
Results: A total of 244 patients received LDP/CDP. Most patients reported AEs that were non-serious (n=181, 74.2%) and mild (n=50, 20.5%) to moderate (n=117, 48.0%) in severity. When evaluated over time, the proportion of patients who reported infusion site AEs and systemic AEs was higher in the 4-week optimization period than during the maintenance period intervals [figure1]. A majority of patients who discontinued LDP/CDP (n/N=66/107, 61.7%) did so within the first 10 weeks of treatment [table1].
Conclusion: Most of the adverse events and discontinuations occurred within the first 10 weeks of the study (including during the 4-week optimization period), which was the time period healthcare providers and patients were learning about the delivery system. Patient education before and during LDP/CDP treatment may help to minimize AEs, thereby improving treatment adherence.
To cite this abstract in AMA style:
D. Kern, S. Isaacson, S. Dhanani, J. Aldred, B. Bergmans, D. Santos-Garcia, T. Oeda, F. Gandor, P. Kukreja, L. Bergmann, R. Gupta, S. Talapala, A. Spiegel, T. Kimber. Safety of foslevodopa/foscarbidopa during optimization and maintenance treatment: Post hoc analysis of a phase 3, single-arm trial [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/safety-of-foslevodopa-foscarbidopa-during-optimization-and-maintenance-treatment-post-hoc-analysis-of-a-phase-3-single-arm-trial/. Accessed January 5, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-of-foslevodopa-foscarbidopa-during-optimization-and-maintenance-treatment-post-hoc-analysis-of-a-phase-3-single-arm-trial/