Category: Parkinson’s Disease: Clinical Trials
Objective: Present the efficacy and safety of 24-hour/day continuous subcutaneous infusion (CSCI) of foslevodopa/foscarbidopa (LDP/CDP) across pre-specified subgroups (age, sex, body mass index [BMI], race, duration of PD, and dopamine agonist use) of patients with advanced Parkinson’s disease (aPD) in a phase 3 study.
Background: Continuous delivery of LDP/CDP achieves stable, individualized plasma levodopa concentrations in patients with aPD. In a phase 3, double-blind, active-controlled, multicenter study, superiority of LDP/CDP over oral levodopa (LD)/carbidopa (CD) was demonstrated by a significant increase in “On” time without troublesome dyskinesia (model-based mean [SE] 2.72 [0.52] vs 0.97 [0.50] hours) and reduction in “Off” time (-2.75 [0.50] vs -0.96 [0.49] hours) (NCT04380142) [1].
Method: This analysis compared patients across subgroups including age (< 65 years, ≥ 65 years), sex (male, female), BMI (< 25 kg/m2, ≥ 25 kg/m2), race (White, Other), duration of PD (< 10 years, ≥ 10 years), and dopamine agonist use (yes, no). Analyses of change from baseline to final visit were conducted for the primary endpoint, “On” time without troublesome dyskinesia, and the first key secondary endpoint, “Off” time, as assessed by PD diaries. An ANCOVA model was used for these analyses. Safety was assessed throughout the study.
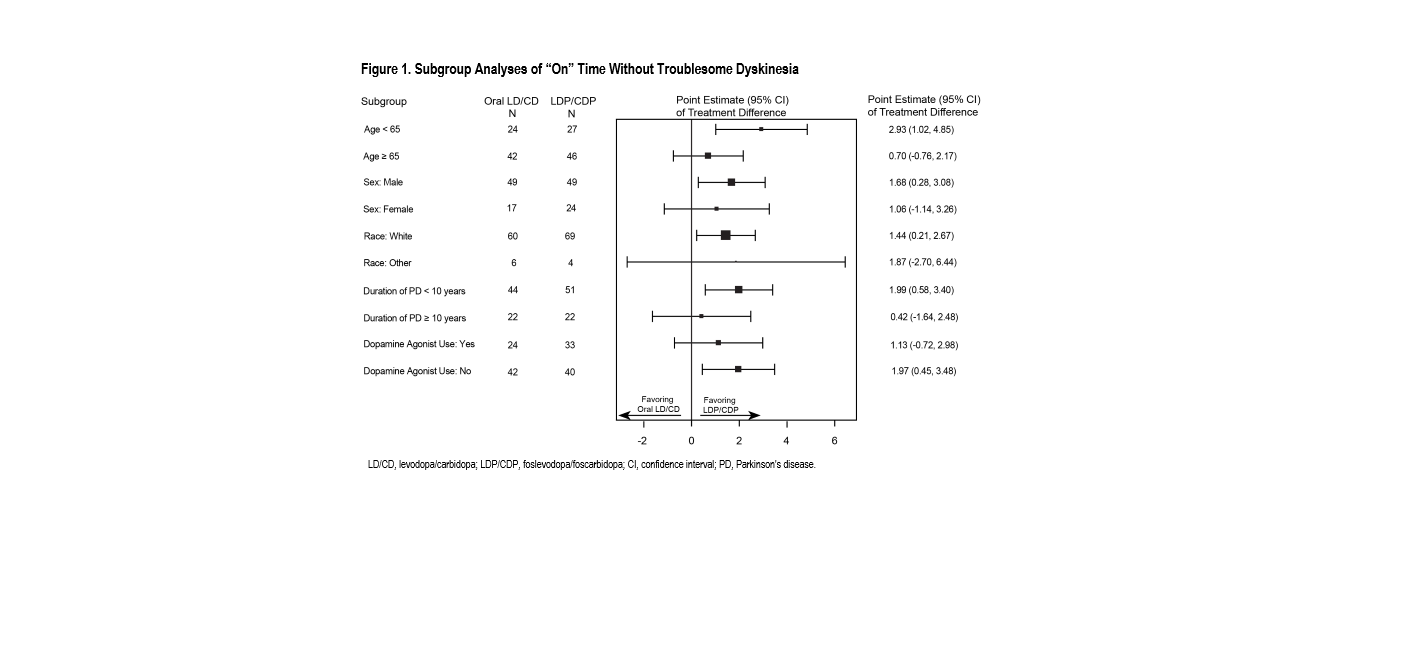
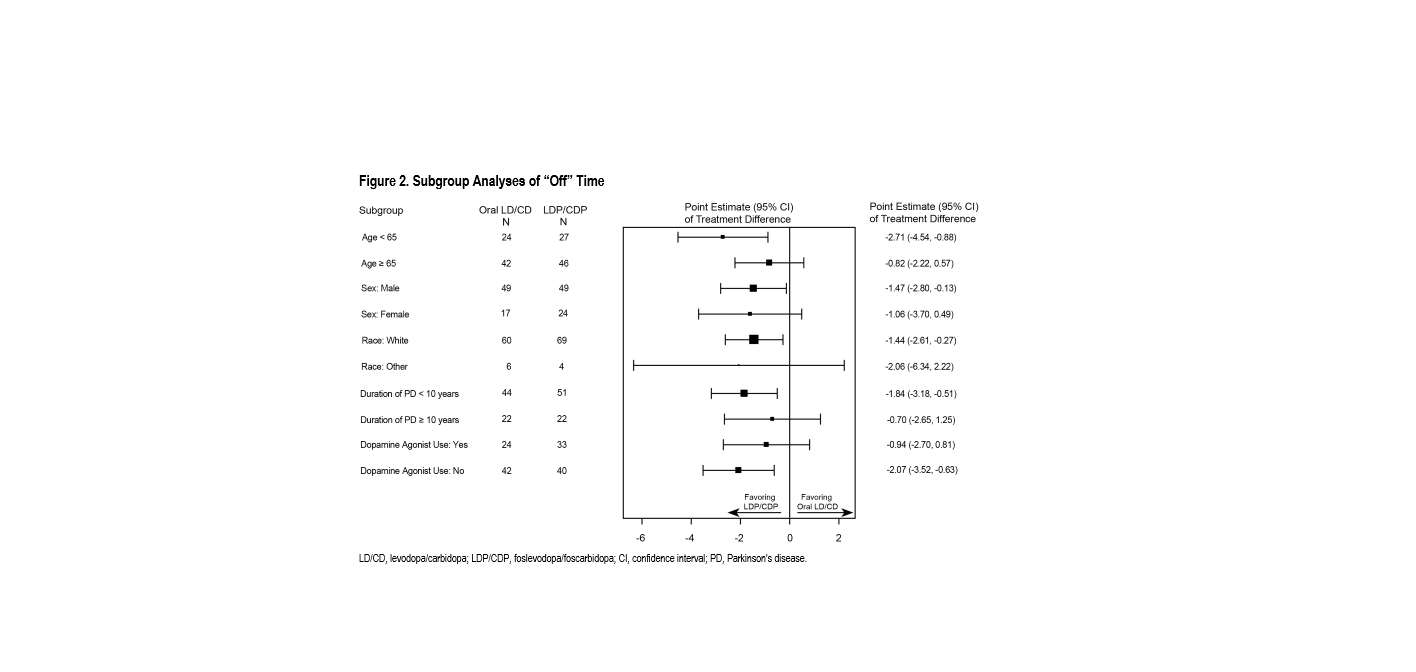
Results: Consistent with the overall study findings, subgroup analyses demonstrated results favoring LDP/CDP over oral LD/CD, with consistent treatment effect across subgroups on the primary and first key secondary efficacy endpoints; a small differential treatment effect was observed for age on the primary efficacy endpoint, but the treatment benefit favored LDP/CDP for both age subgroups [figure 1, figure 2]. No meaningful differences in adverse events were observed by subgroups [table 1].
Conclusion: LDP/CDP provided consistent benefits in “On” time and “Off” time across all pre-specified subgroups, and the LDP/CDP benefit/risk profile in aPD is consistently favorable across subgroups including age, sex, race, duration of PD, and dopamine agonist use.
References: [1] Soileau, M. J. et al. Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol.2022; 21:1099–1109.
To cite this abstract in AMA style:
M. Soileau, N. Fisseha, A. Jeong, T. Kimber, K. Klos, W. Robieson, S. Talapala, E. Vaou, H. Zheng, M. Facheris, R. Hauser. Foslevodopa/foscarbidopa in patients with advanced Parkinson’s disease: subgroup analyses from a phase 3, randomized study [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/foslevodopa-foscarbidopa-in-patients-with-advanced-parkinsons-disease-subgroup-analyses-from-a-phase-3-randomized-study/. Accessed January 8, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/foslevodopa-foscarbidopa-in-patients-with-advanced-parkinsons-disease-subgroup-analyses-from-a-phase-3-randomized-study/