Objective: To examine onabotulinumtoxinA (onabotA) treatment utilization patterns in the United States (US) for pronated forearm and intrinsic plus hand postures in patients with upper limb (UL) spasticity over a 2-year period.
Background: In 2021, onabotA received approval in the US for injection into an additional 8 muscles in the treatment of adult UL spasticity including muscles of the elbow and forearm (brachialis, brachioradialis, pronator teres, and pronator quadratus), as well as intrinsic hand muscles (lumbricals and interossei) and thumb muscles (flexor pollicis brevis and opponens pollicis). More information is required to maximize effectiveness in patients with UL spasticity.
Method: This post hoc analysis utilized US data from the multicenter, prospective, observational Adult Spasticity International Registry (ASPIRE) study (NCT01930786)
which ran from 2013 through 2017. Participants were adult patients who received onabotA (total dose ≤400 U per treatment session) to UL muscles for spasticity. This
analysis was centered on the 2021 onabotA expanded label and evaluated treatment utilization for pronated forearm and intrinsic plus hand postures in patients with UL
spasticity to determine if there is an educational opportunity for optimized treatment in the real-world setting.
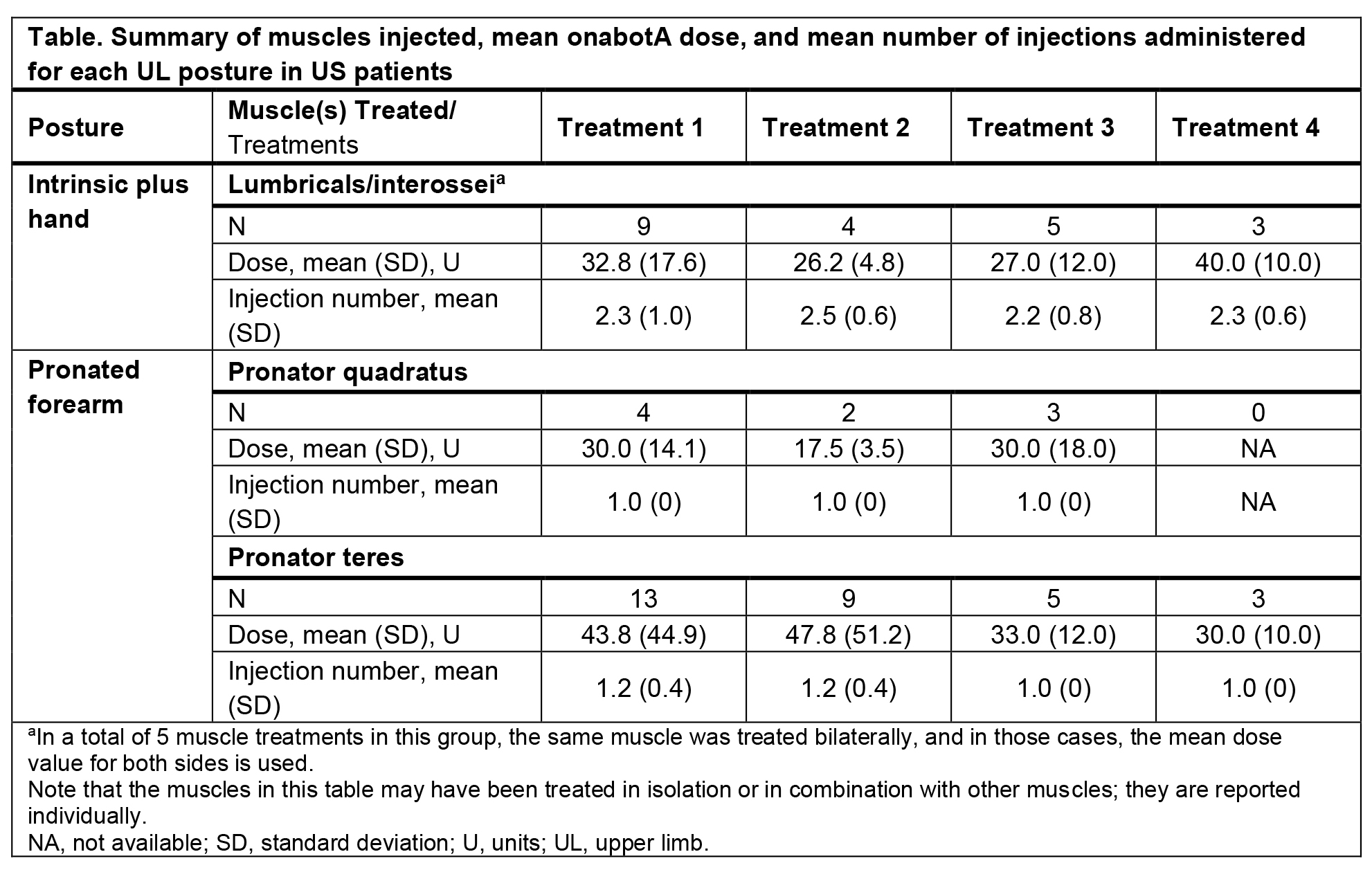
Results: Of 316 US patients in ASPIRE treated for UL spasticity, 135/316 (43%) had pronated forearm and 89/316 (28%) had intrinsic plus hand postures. OnabotA (≤400
U) treatment was delivered to select UL muscles of 45 patients consistent with the expanded US label. Baseline characteristics of these 45 patients included a mean age
(SD) of 51.3 (16.0) years, 23/45 (51%) who were onabotA-naive, and 26/45 (58%) were male. New postures treated included intrinsic plus hand (9/45; 20.0%) and pronated
forearm (14/45; 31.1%).[table1] Of the 38 adverse events (AEs) that occurred, 1 was treatment-related (muscular weakness) and no treatment-related serious AEs were
reported.
Conclusion: This analysis provides real-world evidence of the opportunity to educate on the treatment of pronated forearm and intrinsic plus hand postures in accordance with the expanded label for onabotA.
To cite this abstract in AMA style:
G. Francisco, W. Feng, M. Munin, K. Ngo, M. Schwartz, M. Sadeghi, A. Zuzek, A. Esquenazi. Treating Upper Limb Spasticity With OnabotulinumtoxinA: Subgroup Analysis of US Practice Patterns From the ASPIRE Study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/treating-upper-limb-spasticity-with-onabotulinumtoxina-subgroup-analysis-of-us-practice-patterns-from-the-aspire-study/. Accessed April 2, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/treating-upper-limb-spasticity-with-onabotulinumtoxina-subgroup-analysis-of-us-practice-patterns-from-the-aspire-study/