Category: Parkinson's Disease: Neurophysiology
Objective: The present study aims to demonstrate a possible application of the analysis of functional brain connectivity from brain connectivity matrices.
Specifically, we aim to:
– Differentiate between the healthy gait state and the involuntary freezing state in Parkinson’s disease
– to show that our methodology is also capable of differentiate the voluntary rest state and the involuntary freezing state
Background: Freezing of gait (FOG) is the most common gait disorder in advanced Parkinson’s disease (PD), with a great impact on the quality of life and morbidity of these patients. Current therapies are partially effective, likely reflecting their multifaceted and enigmatic underlying pathophysiology. One of the strengths of Electroencephalography (EEG) is its ability to assess brain function while walking; Its millisecond temporal resolution may allow more accurate study of brain dynamics during freezing episodes.
Method: In this experimental study, 3 patients with Parkinson’s Disease and FOG, in off medical, performed two series of timed up and go (TUG) tasks: Regular TUG and trials including voluntary stops with verbal instructions to suddenly stop movement. The EEG time series of each individual has moments of involuntary freezing state, healthy gait state and voluntary rest state. Brain connectivity matrices were used, using Pearson correlations, Spearman correlations, Granger causality and mutual information to feed a machine learning algorithm.
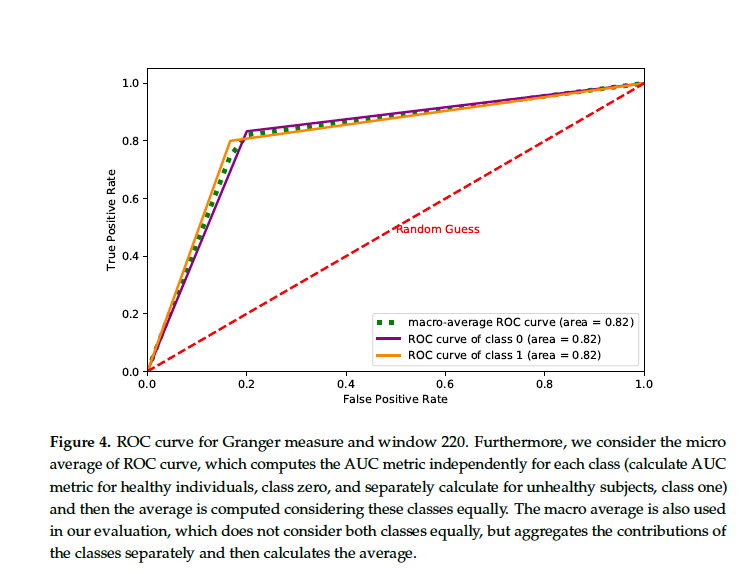
Results: In the most cases, the connectivity matrix method presents excellent performance in discriminating patients in different brain states. We obtained the results in the Table 1. In Figure 4, we calculated the area under the curve (AUC) (1.0) = 0.83 for the naive bayes classifier. In all cases, our tests showed that the use of connectivity matrices is a reliable technique for differentiating distinct brain information.
Conclusion: This study adds relevant insights into possible changes in brain connectivity in FOG. Differentiating brain connectivity from FOG and voluntary arrest may help in future brain-machine interface technologies and in the prognostic assessment of future rehabilitations in FOG.
References: [1] Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017 Mar 23;3:17013
[2] Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015 Aug 29;386(9996):896-912. doi: 10.1016/S0140-6736(14)61393-3
[3] Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23 Suppl 2:S423-5. doi: 10.1002/mds.21927. Erratum in: Mov Disord. 2008 Aug 15;23(11):1639-40. PMID: 18668629.
[4] J.G. Nutt, B.R. Bloem, N. Giladi, M. Hallet, F.B. Horak, and A. Niewboer, E.H. Miller, ”Freezing of gait: moving forward on a mysterious clinical phenomenon”, vol. 10, pp. 2011, The Lancet Neurology, pp.734-744, 2011
[5] Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, Tanner C; Parkinson Study Group. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001 Jun 26;56(12):1712-21
[6] Walton CC, Shine JM, Hall JM, O’Callaghan C, Mowszowski L, Gilat M, Szeto JY, Naismith SL, Lewis SJ. The major impact of freezing of gait on quality of life in Parkinson’s disease. J Neurol. 2015 Jan;262(1):108-15. doi: 10.1007/s00415-014-7524-3. Epub 2014 Oct 16. PMID: 25319020
[7] Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004 Aug;19(8):871-84
[8] J.M. Shine, S.L. Naishmith, and S.J.G. Lewis, ”The pathophysiological mechanisms underlying freezing of gait in Parkinson’s disease”, Journal of Clinical Neuroscience, vol. 18, pp. 1154-1157, 2011
[9] Walton CC, Shine JM, Mowszowski L, Naismith SL, Lewis SJ. Freezing of gait in Parkinson’s disease: current treatments and the potential role for cognitive training. Restor Neurol Neurosci. 2014;32(3):411-22.
[10] Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson’s disease: where are we now? Curr Neurol Neurosci Rep. 2013 Jun;13(6):350.
[11] Nieuwboer A, Giladi N. Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Mov Disord. 2013 Sep 15;28(11):1509-19.
[12] Bharti, K., Suppa, A., Pietracupa, S. et al. Aberrant functional connectivity in patients with Parkinson’s disease and freezing of gait: a within- and between-network analysis. Brain Imaging and Behavior 14, 1543–1554 (2020)
[14] Logothetis, N.K., J. Pauls, M. Augath, T. Trinath and A. Oeltermann,“Neurophysiological investigation of the basis of the fMRI signal,” in Nature, vol. 412, no. 6843, 2001, pp. 150–157.
[15] Pozzi, N.G.; Canessa, A.; Palmisano, C.; Brumberg, J.; Steigerwald, F.; Reich, M.M.; Minafra, B.; Pacchetti, C.; Pezzoli, G.; Volkmann, J.; others. Freezing of gait in Parkinson’s disease reflects a sudden derangement of locomotor network dynamics. Brain 2019, 142, 2037–2050.
[16] Handojoseno, A.A.; Shine, J.M.; Gilat, M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.; Nguyen, H.T. Prediction of freezing of gait using analysis of brain effective connectivity. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, 2014, pp. 4119–412
[17] Fallani, F.D.V.; da Fontoura Costa, L.; Rodriguez, F.A.; Astolfi, L.; Vecchiato, G.; Toppi, J.; Borghini, G.; Cincotti, F.; Mattia, D.; Salinari, S.; others. A graph-theoretical approach in brain functional networks. Possible implications in EEG studies. Nonlinear biomedical physics. Springer, 2010, Vol. 4, pp. 1–13.
[18] Delval, A.; Bayot, M.; Defebvre, L.; Dujardin, K. Cortical oscillations during gait: wouldn’t walking be so automatic? Brain sciences 2020, 10, 90.
[19] Luu, T.P.; Nakagome, S.; He, Y.; Contreras-Vidal, J.L. Real-time EEG-based brain-computer interface to a virtual avatar enhances cortical involvement in human treadmill walking. Scientific reports 2017, 7, 1–12.
[20] Zach H, Janssen AM, Snijders AH, et al. Identifying freezing of gait in Parkinson’s disease during freezing provoking tasks using waist-mounted accelerometry. Parkinsonism Relat Disord 2015; 21:1362–1366.
[21] Bachlin M, Plotnik M, Roggen D, et al. Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans Inf Tech Biomed 2010;14:436–446.
[22] lyasova, N.; Kupriyanov, A.; Paringer, R.; Kirsh, D. Particular use of BIG DATA in medicaldiagnostic tasks. Pattern Recognition and Image Analysis 2018, 28, 114–121.
[23] Richens, J.G.; Lee, C.M.; Johri, S. Improving the accuracy of medical diagnosis with causal machine learning. Nature communications 2020, 11, 1–9.
[24] ynch, C.J.; Liston, C. New machine-learning technologies for computer-aided diagnosis. Nature medicine 2018, 24, 1304–1305.
[25] Vanegas, M.I.; Ghilardi, M.F.; Kelly, S.P.; Blangero, A. Machine learning for EEG-based biomarkers in Parkinson’s disease. 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). IEEE, 2018, pp. 2661–2665.
To cite this abstract in AMA style:
D. Salgado, A. Pineda, C. Alves, R. Carra, F. Rodrigues, M. Souza, R. Cury. Connectivity matrices for differentiating voluntary stopping states and involuntary freezing states in Parkinson’s disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/connectivity-matrices-for-differentiating-voluntary-stopping-states-and-involuntary-freezing-states-in-parkinsons-disease/. Accessed December 13, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/connectivity-matrices-for-differentiating-voluntary-stopping-states-and-involuntary-freezing-states-in-parkinsons-disease/