Objective: We analyzed changes in the concentration of primary metabolites in post-mortem brain samples from FXTAS cases. We also investigated how these changes relate to the levels of fragile X mental retardation protein (FMRP), to the number of CGG repeats, and to the presence of the APOE ε4 allele; as well as to the clinical phenotype – tremor, ataxia, dementia and parkinsonism.
Background: Studies conducted in blood samples collected from FMR1 premutation carriers with FXTAS reported glucose shunt and abnormalities in energy metabolism [1,2,3]. Some metabolites showed the potential to become biomarkers for carriers developing FXTAS [4]. In addition, individuals with the APOE ε4 allele, display metabolic changes decades before the onset of neurological symptoms in Alzheimer’s Disease [5]; this may contribute as a genetic factor predisposing to the development of FXTAS [6].
Method: We evaluated 20 brains with FXTAS and 20 matched controls and compared primary metabolites concentration. Concentrations were determined using gas chromatography mass spectrometric quantification using parameters as previously reported [7]. CGG repeat size was determined by Southern blot and PCR analysis and FMRP levels were determined by Western Blot. Genotyping for assessing APOE allelic variants was carried out using TaqMan®. Metabolite data were log transformed and cyclic loess normalized prior to analysis. Metabolite concentrations were compared between groups using the Bioconductor package limma [8], and a model that included group, age, and PMI.
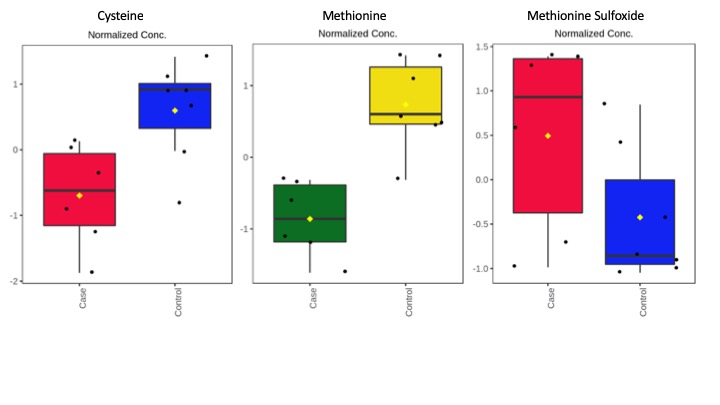
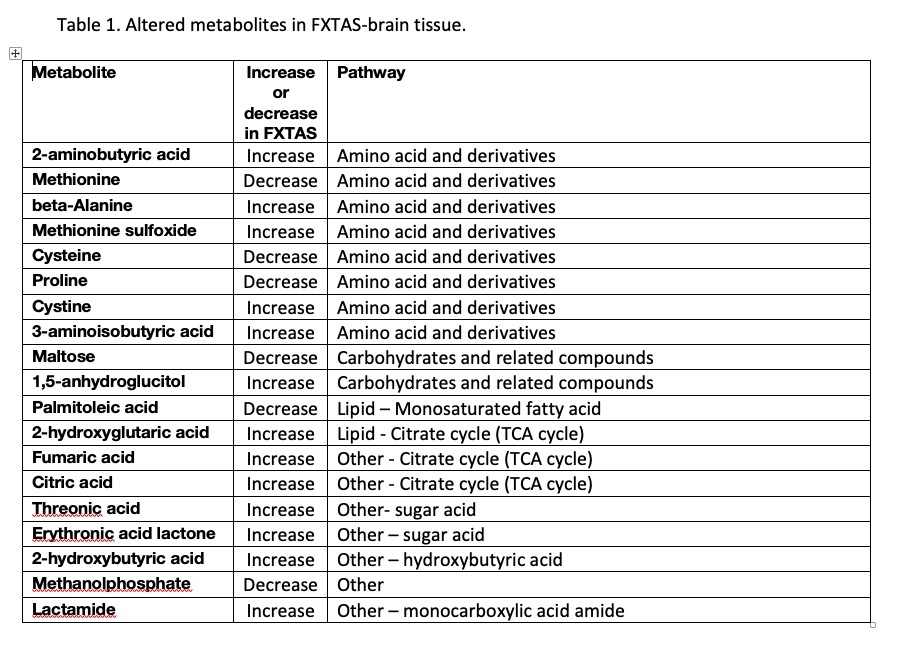
Results: Preliminary untargeted analysis detected 513 primary metabolites, out of which, 149 were identified. 19 metabolites showed a trending difference between groups (unadj P<0.05). Metabolites were classified based on their biological roles. The preliminary list of metabolites of interest is found in [table1]. A handful of metabolites involved in amino acids metabolism showed alterations as well compounds involved in the TCA cycle. Alterations in some of the listed metabolites were found in other neurodegenerative diseases [9,10] and in plasma from carriers developing FXTAS [4]. The potential correlation with symptoms of FXTAS and molecular variables is currently under analysis.
Conclusion: Our data identified alterations in metabolic pathways related to oxidative-stress response and bioenergetics in brains with FXTAS suggesting their potential role in the pathogenesis of the disease.
References: [1] Napoli E, et al. Warburg effect linked to cognitive-executive deficits in FMR1 premutation. FASEB J. 2016;30(10):3334-3351.
[2] Giulivi C, et al. Plasma metabolic profile delineates roles for neurodegeneration, pro-inflammatory damage and mitochondrial dysfunction in the FMR1 premutation. Biochem J. 2016;473(21):3871-3888.
[3] Ross-Inta, C., Omanska-Klusek, A., Wong, S., Barrow, C., Garcia-Arocena, D., Iwahashi, C., Berry-Kravis, E., Hagerman, R. J., Hagerman, P. J., & Giulivi, C. (2010). Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. The Biochemical journal, 429(3), 545–552.
[4] Zafarullah M, et al. Metabolic profiling reveals dysregulated lipid metabolism and potential biomarkers associated with the development and progression of Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). FASEB J. 2020;34(12):16676-16692.
[5] Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284-289.
[6] Silva F, et al. High apolipoprotein E4 allele frequency in FXTAS patients. Genet Med. 2013;15(8):639-642.
[7] Fiehn O, et al. Quality control for plant metabolomics: reporting MSI-compliant studies. The Plant journal : for cell and molecular biology. 2008;53(4):691-704.
[8] Ritchie, M.E., Phipson, B., Wu, D., Hu, Y., Law, C.W., Shi, W., and Smyth, G.K. (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research 43(7), e47.
[9] Xu J, Begley P, Church SJ et al., “Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: snapshot of a pervasive metabolic disorder,” Biochimica et Biophysica Acta—Molecular Basis of Disease, vol. 1862, no. 6, pp. 1084–1092, 2016.
[10] Trezzi JP, Galozzi S, Jaeger C, Barkovits K, Brockmann K, Maetzler W, Berg D, Marcus K, Betsou F, Hiller K, Mollenhauer B. Distinct metabolomic signature in cerebrospinal fluid in early parkinson’s disease. Mov Disord. 2017 Oct;32(10):1401-1408.
To cite this abstract in AMA style:
M. Salcedo-Arellano, M. Johnson, Y. Hwang, Y. Mclennan, E. Mcbride, P. Juarez, B. Durbin-Johnson, F. Tassone, R. Hagerman, V. Martinez-Cerdeno. Metabolic profile in the brain with Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/metabolic-profile-in-the-brain-with-fragile-x-associated-tremor-ataxia-syndrome-fxtas/. Accessed April 18, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/metabolic-profile-in-the-brain-with-fragile-x-associated-tremor-ataxia-syndrome-fxtas/