Category: Other
Objective: The objective of this study was to study the clinical phenomenology, investigational characteristics, radiological findings and the outcomes at discharge of patients presenting with MDE’s at the tertiary care neurology emergency service in India.
Background: Most of the literatures available on MDE are in the form of case series and case reports. There are few large systematic studies on the MDE’s globally and fewer from Indian subcontinent.
Method: This was a prospective, descriptive, observational study.
Patients presenting to the emergency department with hypokinetic and hyperkinetic MDE were included in the study [2, 3]. Patients with psychogenic movement disorders and those not willing to give consent were excluded from the study. The study period was from April 2019 to June 2021. Written informed consent was taken from all the study participants.
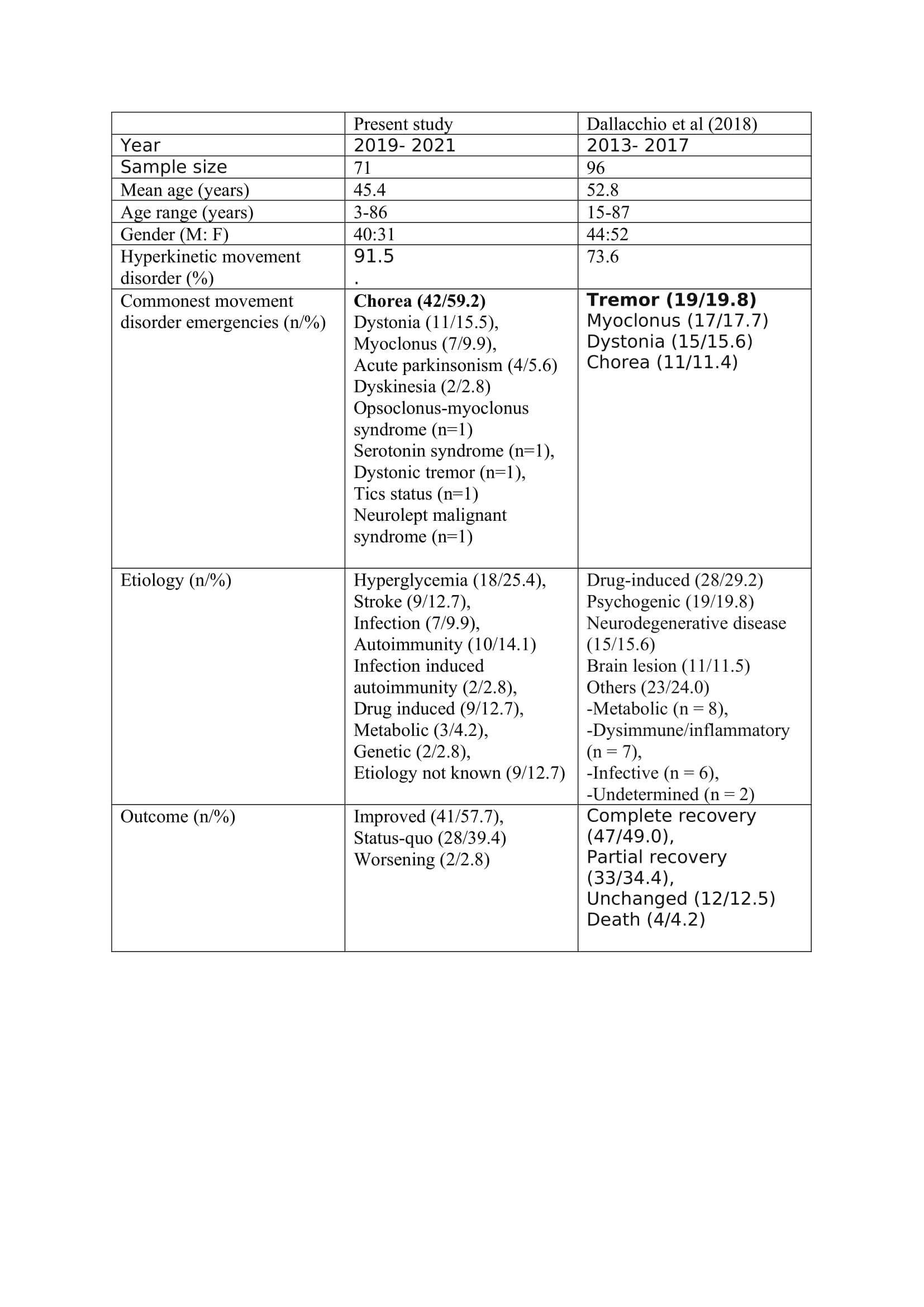
Results: Around 45000 patients with neurological emergencies attended the emergency services during the study period. A total of 90 patients with MDE attended the neurology emergency service. However, 71 patients fulfilling the selection criteria during the study period were included in the final analysis. The mean age of the patients was 45.4 ± 24.4 years (range-3-86 years). Forty patients were male and 31 were females. The mean age of the males was 42.9 ± 21.7 years (range- 3–74 years) and females were 48.8 ± 24.2 years (range- 3–86 years).
Movement disorder emergencies (n=71):
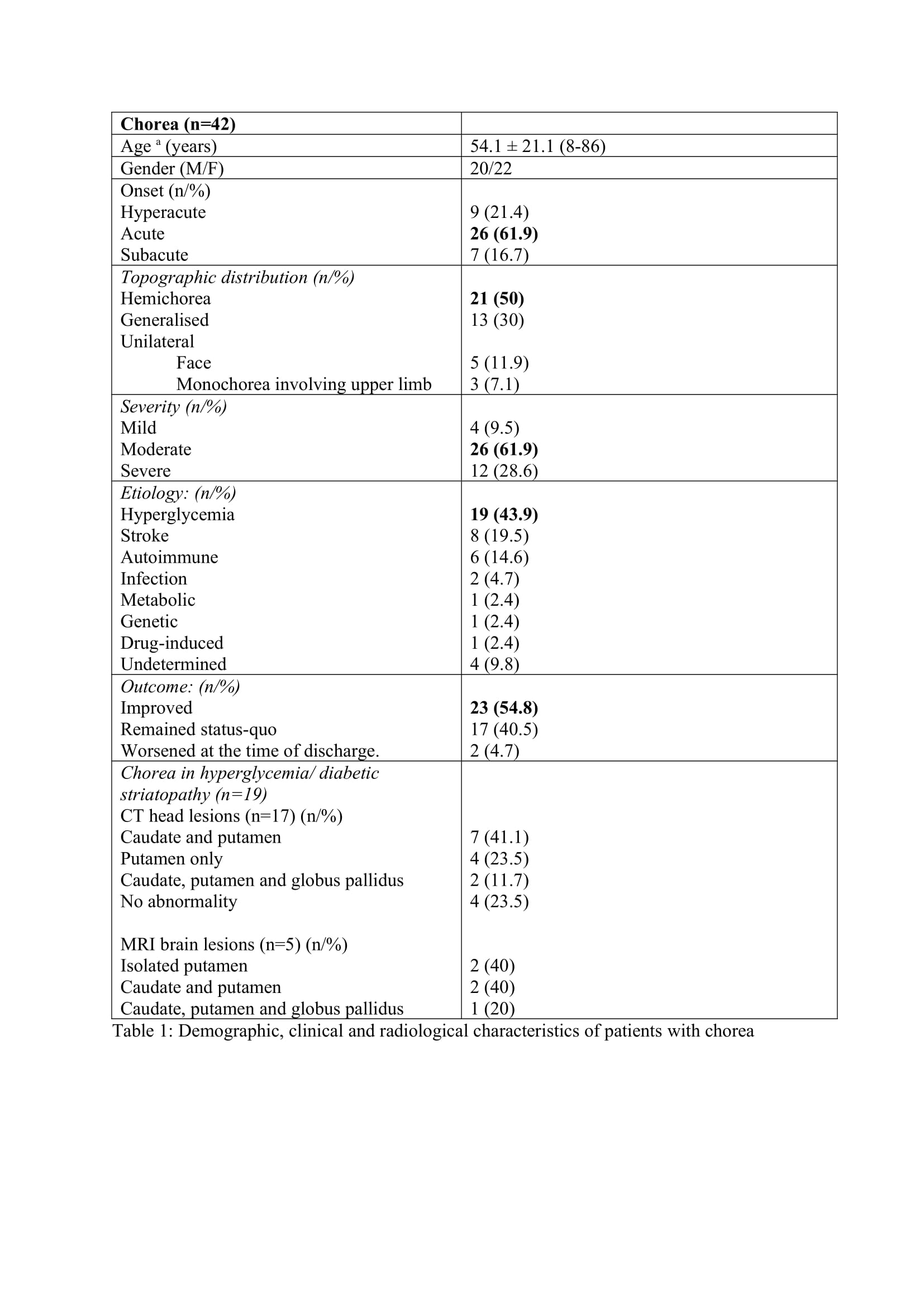
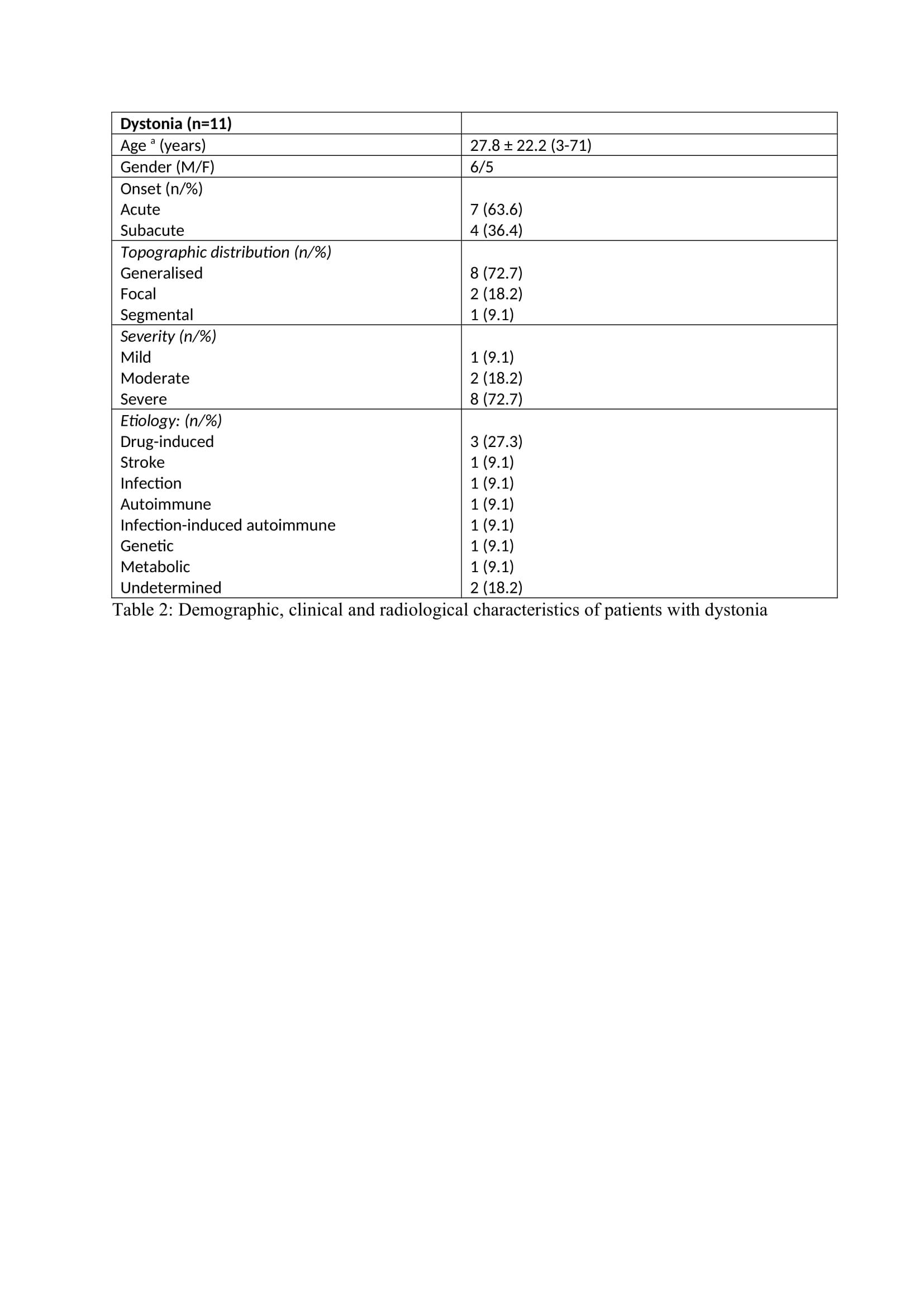
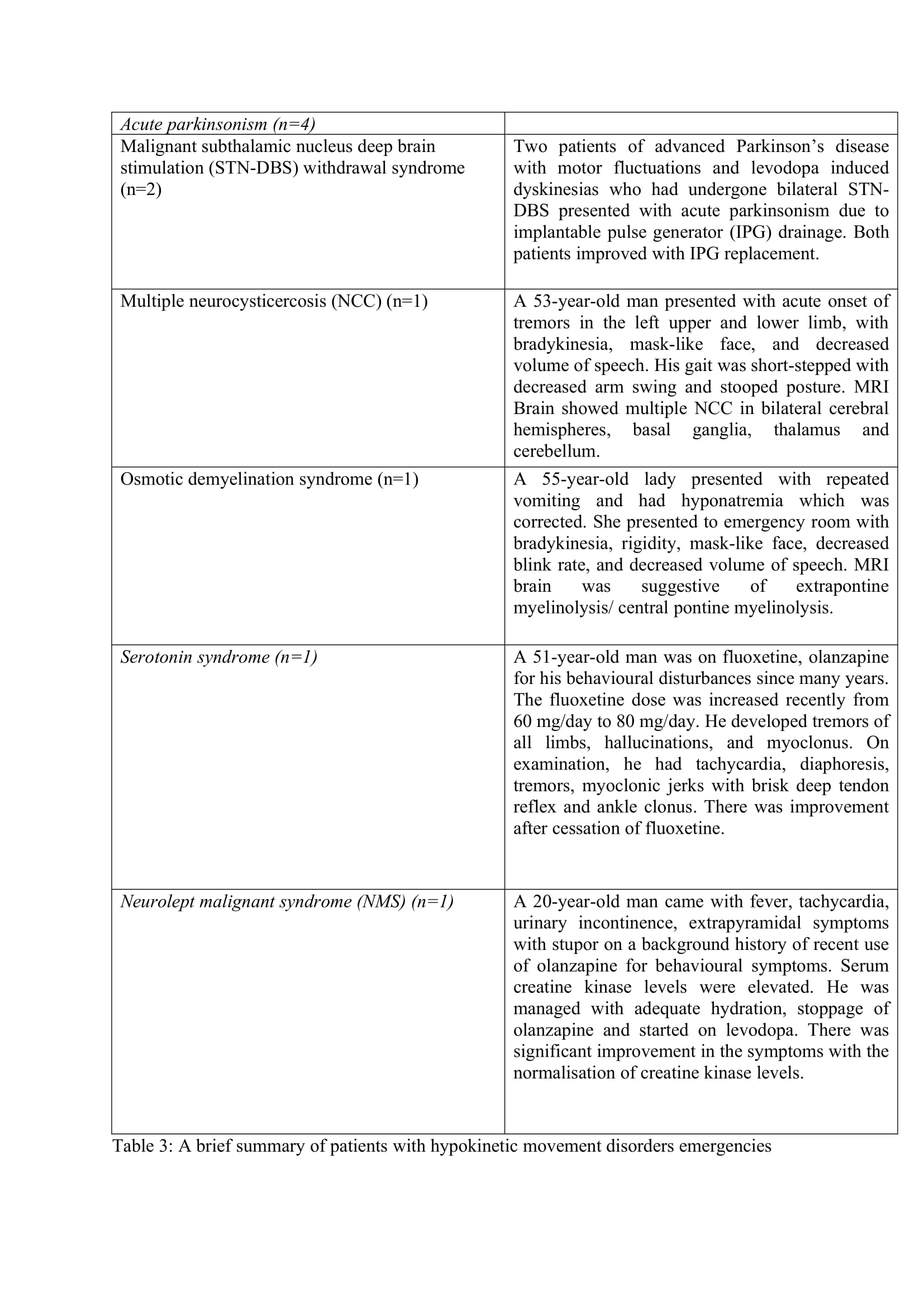
The hyperkinetic MDE (n=65) were predominant (91.5%) than the hypokinetic MDE (n=6). Chorea (n=42) was the predominant hyperkinetic MDE followed by dystonia (n=11), myoclonus (n=7), dyskinesia (n=2), tics (n=1), opsoclonus myoclonus syndrome (n=1) and dystonic tremor (n=1). Four patients had acute parkinsonism and one patient each had NMS and serotonin syndrome (SS). The symptom onset was acute in 47 patients (67.1%), subacute in 14 patients (20%) and hyperacute in 9 patients (12.7%). The mean time from onset of symptoms to presentation was 4.8 days. The severity of symptoms at presentation was mild in 6 patients (8.6%), moderate in 38 patients (53.5%) and severe in 27 patients (38%). The main etiological factor was hyperglycemia which was seen in 18 patients (25.4%).
Conclusion: Our study brings out some novel findings on the MDE in Indian scenario.
References: 1. Frucht SJ. Treatment of movement disorder emergencies. Neurotherapeutics. 2014;11:208-12.
2. Robottom BJ, Weiner WJ, Factor SA. Movement disorders emergencies. Part 1: hypokinetic disorders. Arch Neurol 2011;68:567–72.
3. Robottom BJ, Factor SA, Weiner WJ. Movement disorders emergencies Part 2: hyperkinetic disorders. Arch Neurol 2011;68:719–24.
4. Dallocchio C, Matinella A, Arbasino C, Arno’ N, Glorioso M, Sciarretta M, et al. Movement disorders in emergency settings: a prospective study. Neurol Sci. 2019;40:133–8.
5. Goraya JS. Acute movement disorders in children: experience from a developing country. J Child Neurol. 2015;30:406-11.
6. Teixeira AL Jr, Maia DP, Cardoso F. UFMG Sydenham’s chorea rating scale (USCRS): reliability and consistency. Mov Disord. 2005;20:585-91.
7. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985;35:73–77.
8. Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–7.
9. Rajan S, Kaas B, Moukheiber E. Movement Disorders Emergencies. Semin Neurol. 2019;39:125-136.
10. Munhoz RP, Scorr LM, Factor SA. Movement disorders emergencies. Curr Opin Neurol. 2015;28:406-12.
11. Munhoz RP, Moscovich M, Araujo PD, Teive HA. Movement disorders emergencies: a review. Arq Neuropsiquiatr. 2012;70:453-61.
12. Dale RC, Singh H, Troedson C, Pillai S, Gaikiwari S, KozlowskaK. A prospective study of acute movement disorders in children. Dev Med Child Neurol. 2010;52:739-48.
13. Raucci U, Parisi P, Vanacore N, Garone G, Bondone C, Palmieri A, et al. Acute hyperkinetic movement disorders in Italian paediatric emergency departments. Arch Dis Child. 2018;103:790-4.
14. Tandon N, Anjana RM, Mohan V, Kaur T, Afshin A, Ong K, et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Heal. 2018;6:e1352–62.
15. Anchala R, Kannuri NK, Pant H, Khan H, Franco OH, Di Angelantonio E, et al. Hypertension in India: A systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170–7.
16. Abe Y, Yamamoto T, Soeda T, Kumagai T, Tanno Y, Kubo J, et al. Diabetic striatal disease: clinical presentation, neuroimaging, and pathology. Intern Med. 2009;48:1135-41.
17. Chua CB, Sun CK, Hsu CW, Tai YC, Liang CY, Tsai IT. “Diabetic striatopathy”: clinical presentations, controversy, pathogenesis, treatments, and outcomes. Sci Rep. 2020;10(1):1594.
18. Bansil S, Prakash N, Kaye J, Wrigley S, Manata C, Stevens-Haas C, et al. Movement disorders after stroke in adults: a review. Tremor Other Hyperkinet Mov (N Y). 2012;2:tre-02-42-195-1.
19. Defebvre L, Krystkowiak P. Movement disorders and stroke. Rev Neurol (Paris). 2016;172:483-7.
20. Cardoso F. Sydenham’s chorea. Handb Clin Neurol. 2011;100:221-9.
21. Etemadifar M, Salari M, Badiee H, Mirmosayyeb O. Anti-ma2 receptor encephalitis mimicking Huntington chorea. J Res Med Sci. 2017 Mar 15;22:31
22. Revilla FJ, Racette BA, Perlmutter JS. Chorea and jaw-opening dystonia as a manifestation of NeuroBehcet’s syndrome. Mov Disord. 2000;15:741-4.
23. Baizabal-Carvallo JF, Stocco A, Muscal E, Jankovic J. The spectrum of movement disorders in children with anti-NMDA receptor encephalitis. Mov Disord. 2013;28:543-7.
24. Radhakrishnan DM, Goyal V. Levosulpiride-Induced Dystonia: 7 Cases. J Assoc Physicians India. 2018;66:95-6.
25. Pandey S, Shukla T, Mishra A. The Spectrum of Repetitive Behaviors Associated with Subacute Sclerosing Panencephalitis. Mov Disord. 2021;36:497-503.
26. Ebrahimi-Fakhari D, Hildebrandt C, Davis PE, Rodan LH, Anselm I, Bodamer O. The Spectrum of Movement Disorders in Childhood-Onset Lysosomal Storage Diseases. Mov Disord Clin Pract. 2018;5:149-155.
27. Yıldırım M, Köse E, Keçeli AM, Balasar Ö, Şimşek N. Status dystonicus associated with CLN8 disease. Brain Dev. 2021;43:571-5.
28. Sachdev P, Chee KY, Wilson A. Tics status. Aust N Z J Psychiatry. 1996;30:392-6.
29. Rajan R, Krishnan S, Kesavapisharady KK, Kishore A. Malignant Subthalamic Nucleus-Deep Brain Stimulation Withdrawal Syndrome in Parkinson’s Disease. Mov Disord Clin Pract. 2016;3:288-91.
30. Holla VV, Neeraja K, Surisetti BK, Prasad S, Kamble N, Srinivas D, et al. Deep Brain Stimulation Battery Exhaustion during the COVID-19 Pandemic: Crisis within a Crisis. J Mov Disord. 2020;13(3):218-22.
To cite this abstract in AMA style:
A. Bhoyar, R. Yadav, P. Pal, R. Mahale, N. Kamble, V. Holla. Spectrum of Movement Disorder Emergencies in a Tertiary Care Centre in India: A prospective Observational Study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/spectrum-of-movement-disorder-emergencies-in-a-tertiary-care-centre-in-india-a-prospective-observational-study/. Accessed February 1, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/spectrum-of-movement-disorder-emergencies-in-a-tertiary-care-centre-in-india-a-prospective-observational-study/