Objective: Prothena is developing active vaccine candidates targeting α-synuclein (α-syn) epitopes shown in clinical and/or preclinical studies to disrupt synuclein pathology. These vaccines, which target the underlying pathophysiologic hallmarks of Parkinson’s disease (PD), are intended to raise antibody titers against specific α-syn epitopes, resulting in the potential of disease treatment and/or prevention of PD and other synucleinopathies.
Background: PD affects 7–10 million people worldwide. PD and other synucleinopathies are characterized by pathological accumulation of α-syn in both central and peripheral nervous system neurons, resulting in widespread and progressive motor and non-motor symptoms. Currently, there are no approved disease-modifying treatments; however, prasinezumab, a humanized monoclonal antibody targeting the C-terminal region of α-syn, showed signals of efficacy on multiple prespecified secondary and exploratory clinical endpoints in a phase 2 study (PASADENA, NCT03100149).
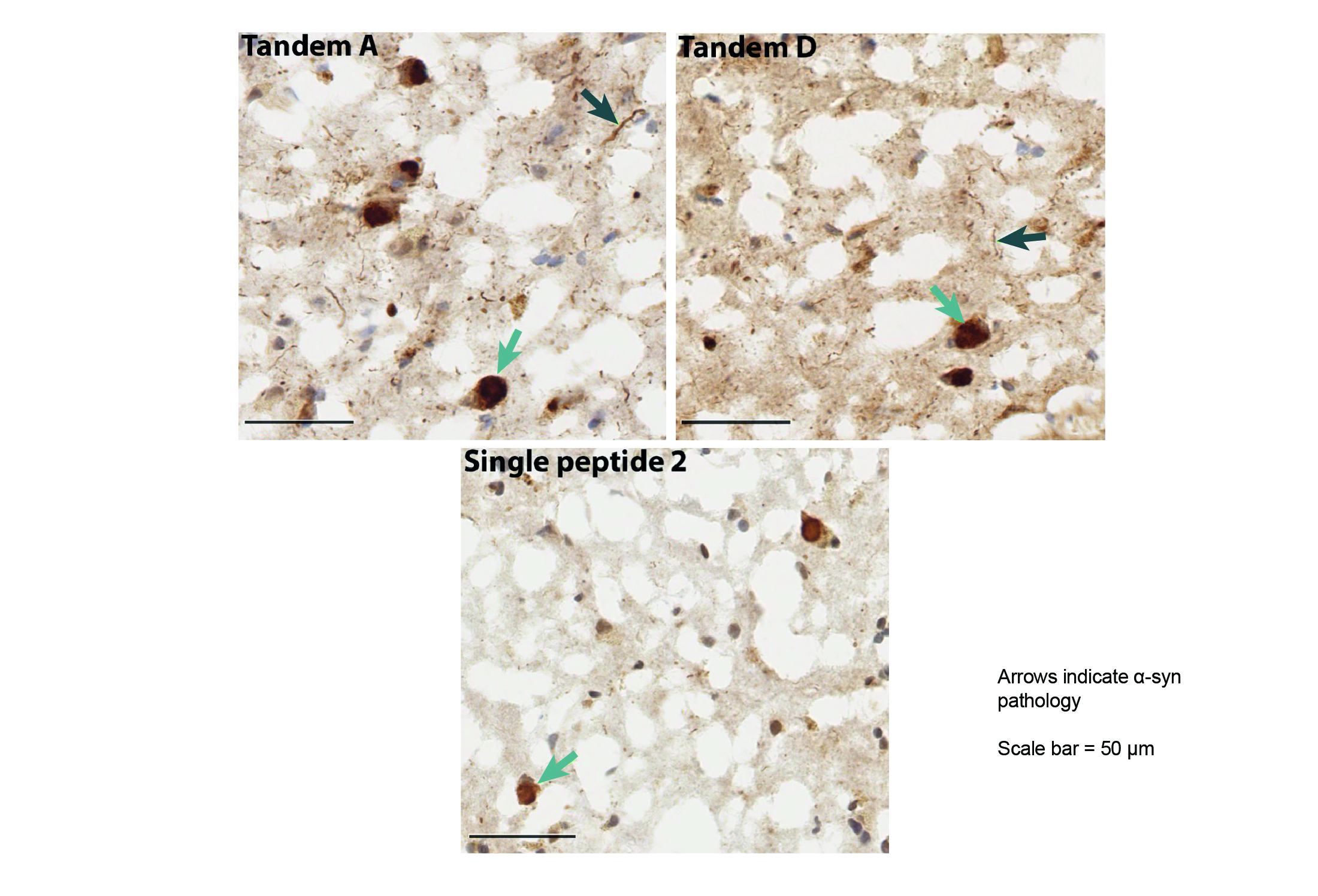
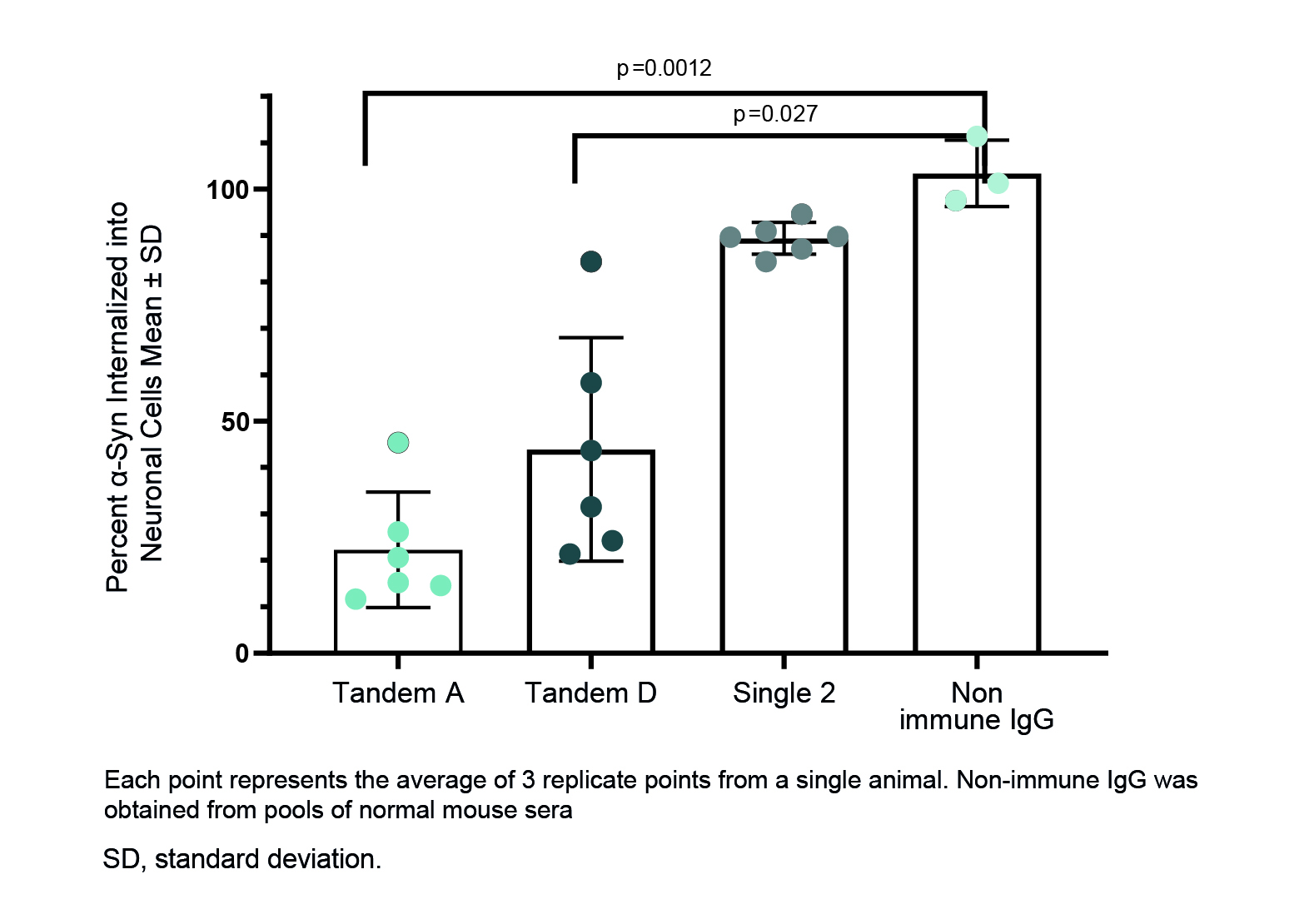
Method: Linear single and tandem α-syn peptides were synthesized and conjugated to CRM 197. Mice were injected with conjugate and QS21 adjuvant. Sera from immunized animals were titered against both recombinant and pre-formed fibrils of α-syn. Fresh frozen PD and control brains were used to confirm specificity of binding to pathological α-syn inclusions. Antibody potency was assessed in vitro by blocking cellular uptake of aggregated α-syn in B103 cells.
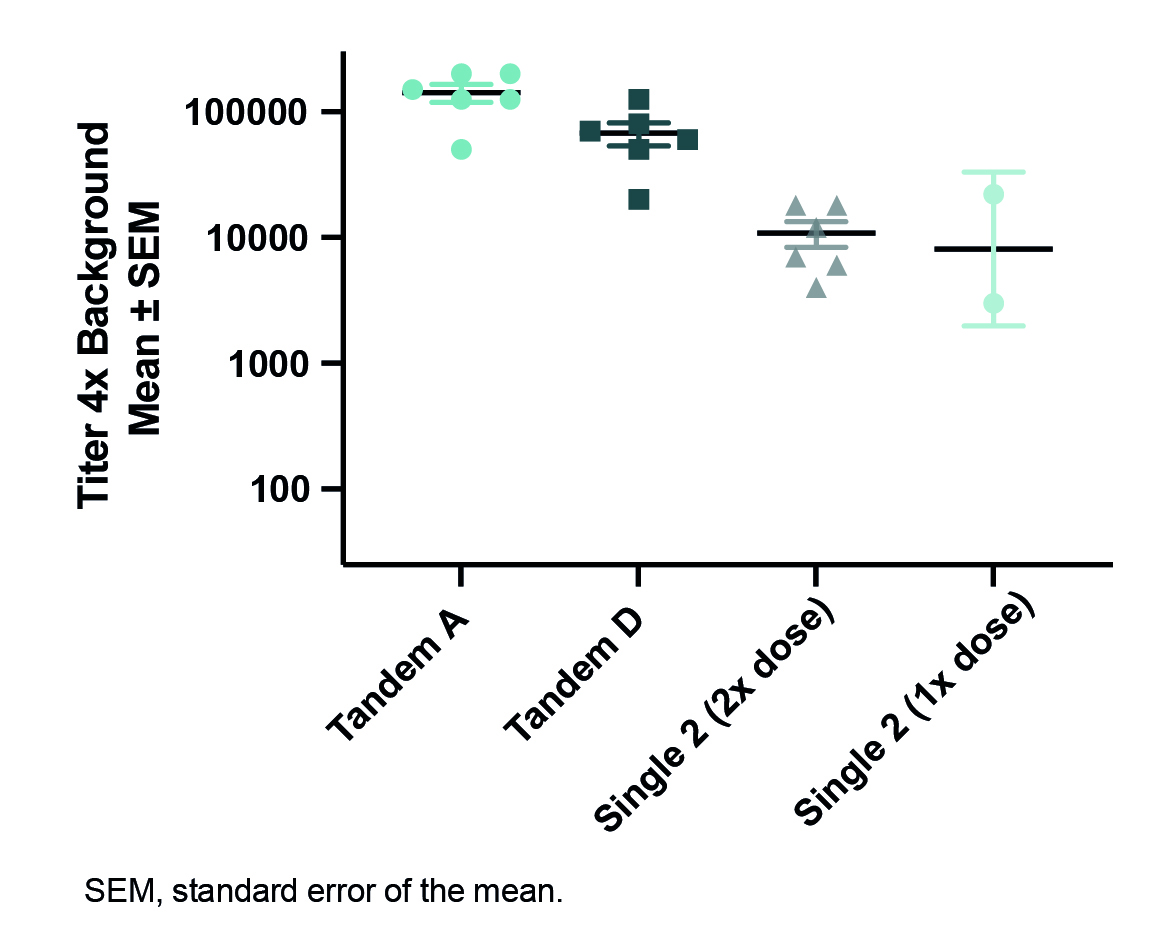
Results: We identified a vaccine candidate with C-terminal α-syn peptides positioned in tandem that produced sera with substantially higher serum titers, greater binding to pathological α-syn inclusions in brains from patients with PD, and a stronger inhibitory activity on the uptake of pathogenic synuclein into cells when compared to antibodies generated with other vaccine constructs (Figures 1-3).
Conclusion: We demonstrated that C-terminal/C-terminal tandem peptide-based vaccine candidates provide superior attributes to both single-peptide vaccines and other tandem peptide vaccines we investigated in all assays: α-syn titers, pathological α-syn staining in human PD brains, and inhibition of α-syn aggregate internalization into a neuronal cell line. These preclinical data support clinical development of multi-peptide vaccines for the potential treatment and prevention of PD and other synucleinopathies.
To cite this abstract in AMA style:
R. Barbour, A. Elmaarouf, L. Louie, S. Tam, C. Tourino, B. Campbell, G. Kinney, W. Zago. Development of C-terminal α-Synuclein Vaccine for Treatment and Prevention of Parkinson’s Disease and Other Synucleinopathies [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/development-of-c-terminal-%ce%b1-synuclein-vaccine-for-treatment-and-prevention-of-parkinsons-disease-and-other-synucleinopathies/. Accessed April 4, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/development-of-c-terminal-%ce%b1-synuclein-vaccine-for-treatment-and-prevention-of-parkinsons-disease-and-other-synucleinopathies/