Category: Parkinson's Disease: Neuroimaging
Objective: To investigate whether intensity of activity and quality of sleep during a 3-month exercise program for patients with Parkinson Disease (PD) are associated with interval change in neuroimaging markers of glymphatic functioning and beta-amyloid (Ab) burden.
Background: PD is a neurodegenerative disease associated with the build-up of alpha-synuclein protein and other metabolic aggregates. Research and clinical practice support exercise as a means to improve cognitive and motor symptoms in PD. A potentially related physiological mechanism for this observation is the glymphatic system, a macroscopic waste-clearance system relying on CSF flow produced by the choroid plexus (CP), driven by arterial pulsation through perivascular spaces, and up-regulated during sleep.
Method: 14 PD patients (12 males; Mage=65.4, SD=6.9) underwent neuroimaging before and after a 3-month exercise program, with actigraph units attached. MRI provided high resolution to quantify parasagittal dural (PSD) space (mL) and choroid plexus (ChP) volume (mL); arterial spin labeling to quantify ChP perfusion (mL/100g/min); phase contrast to quantify CSF flux (mL/min) via the cerebral aqueduct. PET imaging with (11)C-PIB quantified Ab burden (BPND) in regions associated with risk of accumulation in PD variants. Actigraphy (wGT3X-BT) provided in vivo estimates of total time spent in standardized intensity-levels of activity and sleep-state. Non-parametric Spearman correlations were conducted between the actigraph values and pre-post neuroimaging change values.
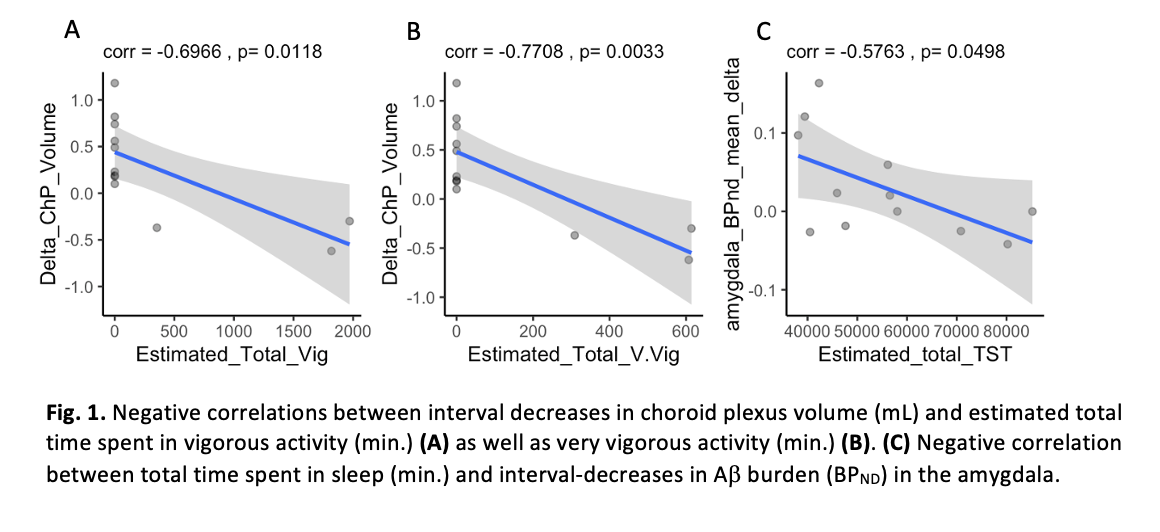
Results: Total time spent in vigorous (r=-0.6966; p=0.0118) and very vigorous (r=-0.7708; p=0.0033) activity was associated with interval-decreases in ChP volume. Total time spent in sleep was associated with interval-decreases in amygdala Ab burden (r=-0.5763; p=0.0498).
Conclusion: Exercise and sleep in PD patients was associated with interval decreases in ChP volume and Ab burden in the amygdala, both suggestive of healthier glymphatic functioning in the context of our prior work. Future work will experimentally control exposure to activity and sleep, as well as replicate in other neurodegenerative conditions.
To cite this abstract in AMA style:
J. Elenberger, J. Eisma, K. Hett, A. Song, C. Mcknight, Y. Yan, H. Kang, M. Donahue, D. Claassen, C. Considine. Activity and sleep associated with changes in glymphatic imaging-markers in Parkinson Disease patients enrolled in 3-month exercise program [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/activity-and-sleep-associated-with-changes-in-glymphatic-imaging-markers-in-parkinson-disease-patients-enrolled-in-3-month-exercise-program/. Accessed February 3, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/activity-and-sleep-associated-with-changes-in-glymphatic-imaging-markers-in-parkinson-disease-patients-enrolled-in-3-month-exercise-program/