Category: Huntington's Disease
Objective: Determine the frequency of comorbidities in people with Huntington’s disease (PwHD) in comparison to non-carrier controls in the Enroll-HD cohort.
Background: Despite some publications suggesting cardiac, muscle, and/or liver involvement, the impact of Huntington’s disease on organs apart from the brain is not well studied. Therapeutics that aim to lower systemic and CNS expression of both the mutant and normal HTT gene are being developed. Since the HTT protein is ubiquitously expressed, it is important to define the systemic pathology in PwHD. Observational studies such as REGISTRY [1] and Enroll-HD [2] have collected comorbidity data from thousands of PwHD and control participants worldwide.
Method: After combining REGISTRY and Enroll-HD data, we used ICD-10 codes to identify comorbidities for each system. PwHD were classified according to the Huntington’s Disease Integrated Staging System (HD-ISS) [3]. The system consists of 4 stages: Stage 0, CAG≥40; Stage 1, caudate and/or putamen atrophy; Stage 2, motor and/or cognitive signs; and Stage 3, functional change. We applied logistic regression, adjusting for age, sex, BMI, and diabetes, to examine the presence of each condition for HD-ISS stages versus controls. Specifically, we examined the occurrence of infectious disease and cancer as well as endocrine, neurological, cardiovascular, pulmonary, gastrointestinal, musculoskeletal, and genitourinary disorders.
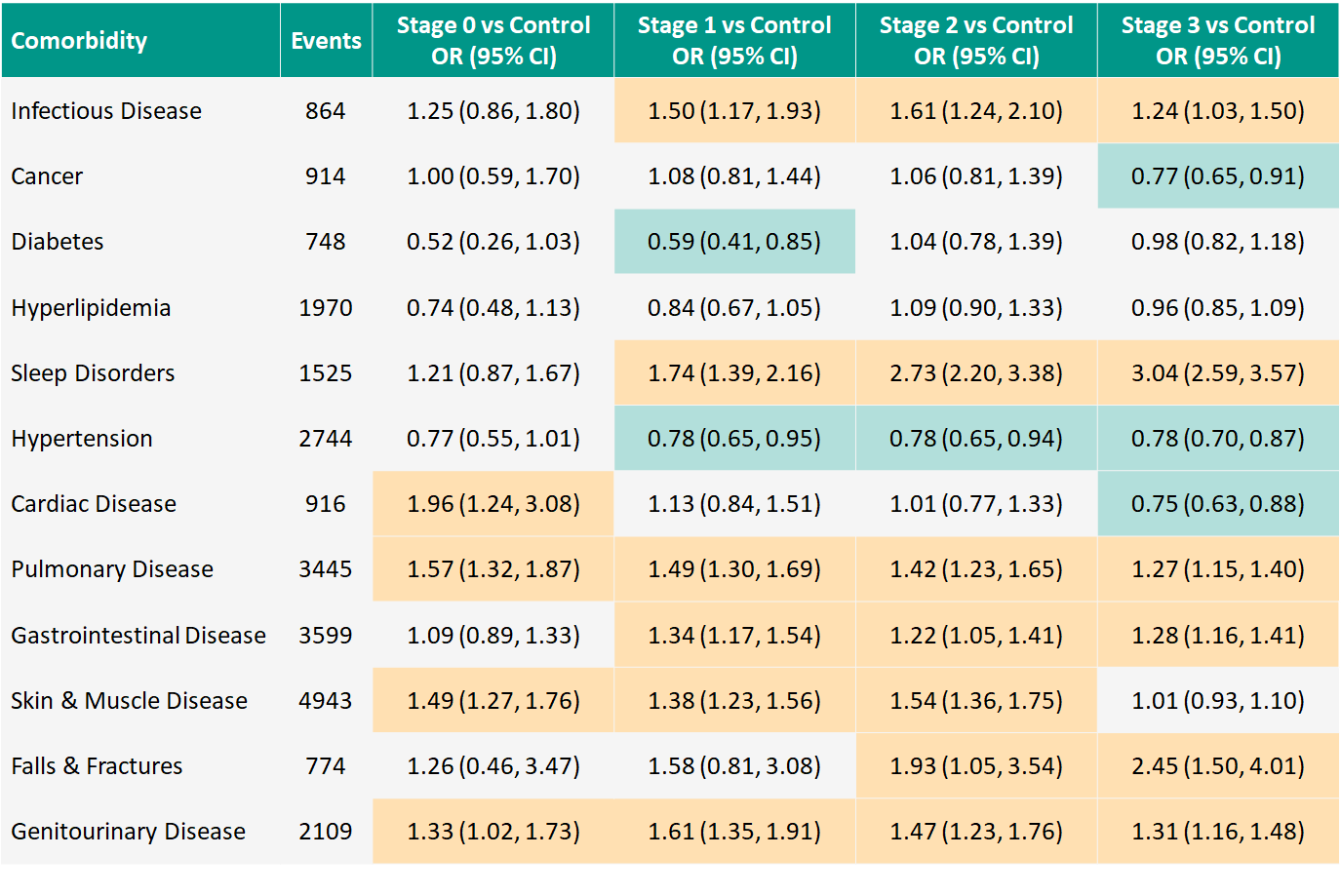
Results: 13,845 PwHD participants (75.2%; mean age 47.7 years, 54% F) and 4559 controls (24.8%; 46.6 years, 62% F) collectively reported 24,551 comorbidities. Sleep disorders, falls, and orthopedic complications are more frequent in PwHD compared to controls. Several conditions appear to have slightly higher frequency for PwHD, including infectious disease and disorders of the pulmonary, gastrointestinal, and genitourinary systems. Some conditions appear less frequently for certain HD-ISS Stages versus controls, notably diabetes (Stage 1) and hypertension (Stage 1-3). [table1]
Conclusion: In contrast to previous reports, our analysis suggests that the frequency of systemic comorbidities is largely similar between PwHD and controls. Further exploration of specific comorbidities such as sleep disorders is warranted. Delineation of systemic comorbidities will help interpret investigational drug-related adverse events. A version of this abstract was accepted as a poster presentation at HDTC, Palm Springs, CA, February 28-March 3, 2022.
References: 1. Orth M, Handley OJ, Schwenke C, Landwehrmeyer B. Observing Huntington’s disease: the European Huntington’s disease network’s REGISTRY. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;82:1409–12.
2. Landwehrmeyer BG, Fitter-Attas C, Giuliano J, et al. Data analytics from Enroll-HD, a global clinical research platform for Huntington’s disease. Movement Disorder Clinical Practice. 2016;4:212–24.
3. Tabrizi SJ, Schobel SA, Gantman EC, et al. Huntington’s Disease Integrated Staging System (HD-ISS): A Novel Evidence-Based Classification System For Staging. Lancet Neurology. 2021.
To cite this abstract in AMA style:
J. Mills, J. Long, J. Vaidya, C. Sampaio, S. Sathe. Comorbidities in Huntington’s disease: An Enroll-HD analysis [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/comorbidities-in-huntingtons-disease-an-enroll-hd-analysis/. Accessed October 21, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/comorbidities-in-huntingtons-disease-an-enroll-hd-analysis/