Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate patterns of concomitant medication use and levodopa equivalent daily dose (LEDD) during a 52 week study with foslevodopa/foscarbidopa (LDP/CDP).
Background: LDP/CDP is a soluble formulation of levodopa/carbidopa prodrugs delivered as continuous subcutaneous infusion (CSCI) 24-hour daily. LDP/CDP provides individually adjustable doses to control motor complications in patients with advanced PD (aPD).
Method: Study M15-741 (NCT03781167) is a 52-week, phase 3, open-label, single-arm, multicenter, global (US, EU, Australia, and Japan) study to assess the local and systemic safety, tolerability, and efficacy of 24-hour daily CSCI of LDP/CDP. The LDP/CDP dose was individualized to achieve an optimal clinical response for each patient. Intake of prior and allowable concomitant medication classes (adamantane derivatives, tropine derivatives, catechol-O-methyltransferase [COMT] inhibitors, monoamine oxidase-B [MAO-B] inhibitors, tertiary amines, other anti-Parkinson drugs, and non-ergolinic dopamine agonists) were summarized by study visit. LEDD for LDP/CDP was evaluated at each study visit.
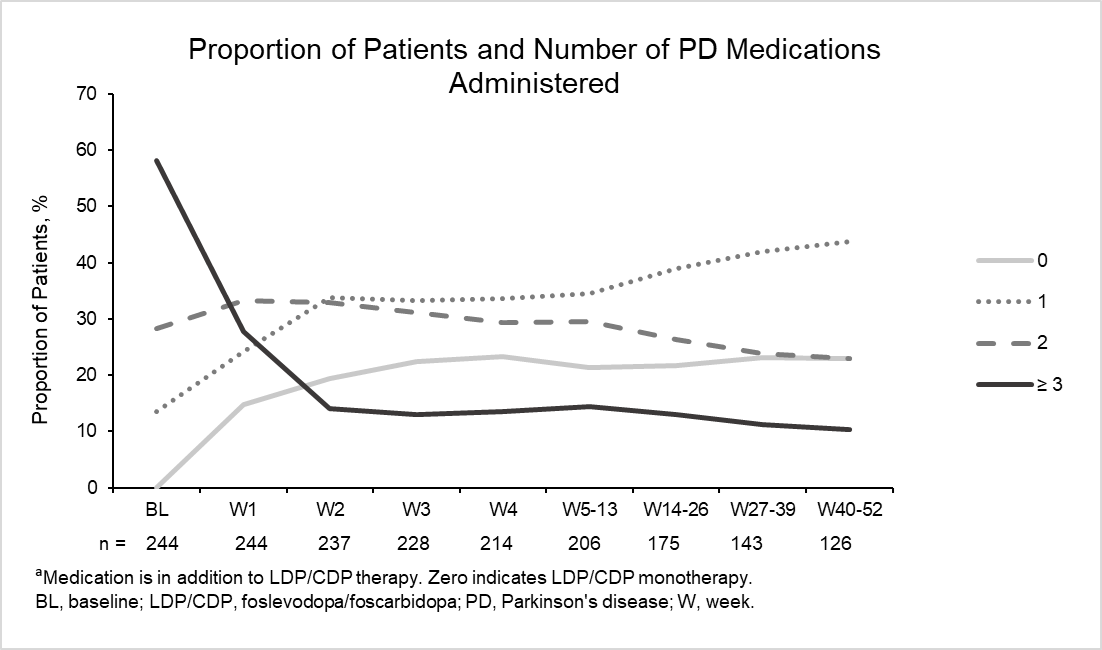
Results: The analysis included 244 patients with aPD. At baseline (BL), the proportion of patients taking 0, 1, 2, and ≥ 3 classes of PD medications were 0%, 13.5%, 28.3%, and 58.2%, respectively. At week 52, there was a reduction in the number of patients taking ≥ 3 PD medications (10.3%) that was associated with a shift in the number of patients taking 0, (LDP/CDP monotherapy; 23.0%), 1 (43.7%) and 2 (23.0%) classes of PD medications (Figure). The LEDD of LDP/CDP (CSCI and extra doses) remained fairly stable over time (range, 1621.9–1847.0 mg/day).
Conclusion: As demonstrated by the treatment patterns observed, there was flexibility in the LDP/CDP dose and use of concomitant medications. For patients with aPD who initiated LDP/CDP treatment, 23% achieved monotherapy with LDP/CDP by week 52. The daily dose of LDP/CDP remained stable over time while the use of concomitant medications decreased, indicating a reduced need for co-medications to adequately control motor fluctuation in patients with aPD.
To cite this abstract in AMA style:
D. Santos Garcia, L. Bergmann, B. Bergmans, F. Bergquist, S. Criswell, V. Fung, J. Jia, P. Kukreja, N. Pavasia, W. Robieson, Z. Zhang, D. Standaert. Concomitant Medication Use and Levodopa Equivalent Daily Dose Requirements After Foslevodopa/foscarbidopa Initiation [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/concomitant-medication-use-and-levodopa-equivalent-daily-dose-requirements-after-foslevodopa-foscarbidopa-initiation/. Accessed December 25, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/concomitant-medication-use-and-levodopa-equivalent-daily-dose-requirements-after-foslevodopa-foscarbidopa-initiation/