Objective: Longitudinal evaluation of personalized intervention strategies informed by detailed clinical and laboratory biomarker analysis to reduce relative risk of Parkinson’s disease in first degree relatives.
Background: Preventing PD is a major unmet need. Over the past decade, a multimodal precision medicine approach has been demonstrated to reduce relative risk of cognitive decline in Alzheimer’s disease (1). The Longitudinal Program to Prevent PD (LoPP-PD) was established to provide personalized multidomain precision medicine interventions to at-risk and high-risk first-degree relatives. Due to the heterogeneity of PD, various models of risk stratifying and subtyping disease based on criteria such as phenotype, pathological features, genetics, and other biomarkers have been proposed, but single biomarkers are unlikely to be sufficient to determine risk and clinical trajectory on an individual basis(2).
Method: Eligible participants must be ≥25 years old with a family history of an alpha-synucleinopathy in a first-degree relative. We established detailed, evidence-based risk profiling for motor, cognitive, and other nonmotor risk using combination of symptoms and biomarkers, including phenotypic symptoms, family history, neurologic examination, anthropometric measurements, genetic analysis, fluid biomarkers, neurocognitive testing, and validated clinical scales. Longitudinal monitoring will be informed by adaptive study design, to allow for ongoing refinement of personalized risk reduction strategies.
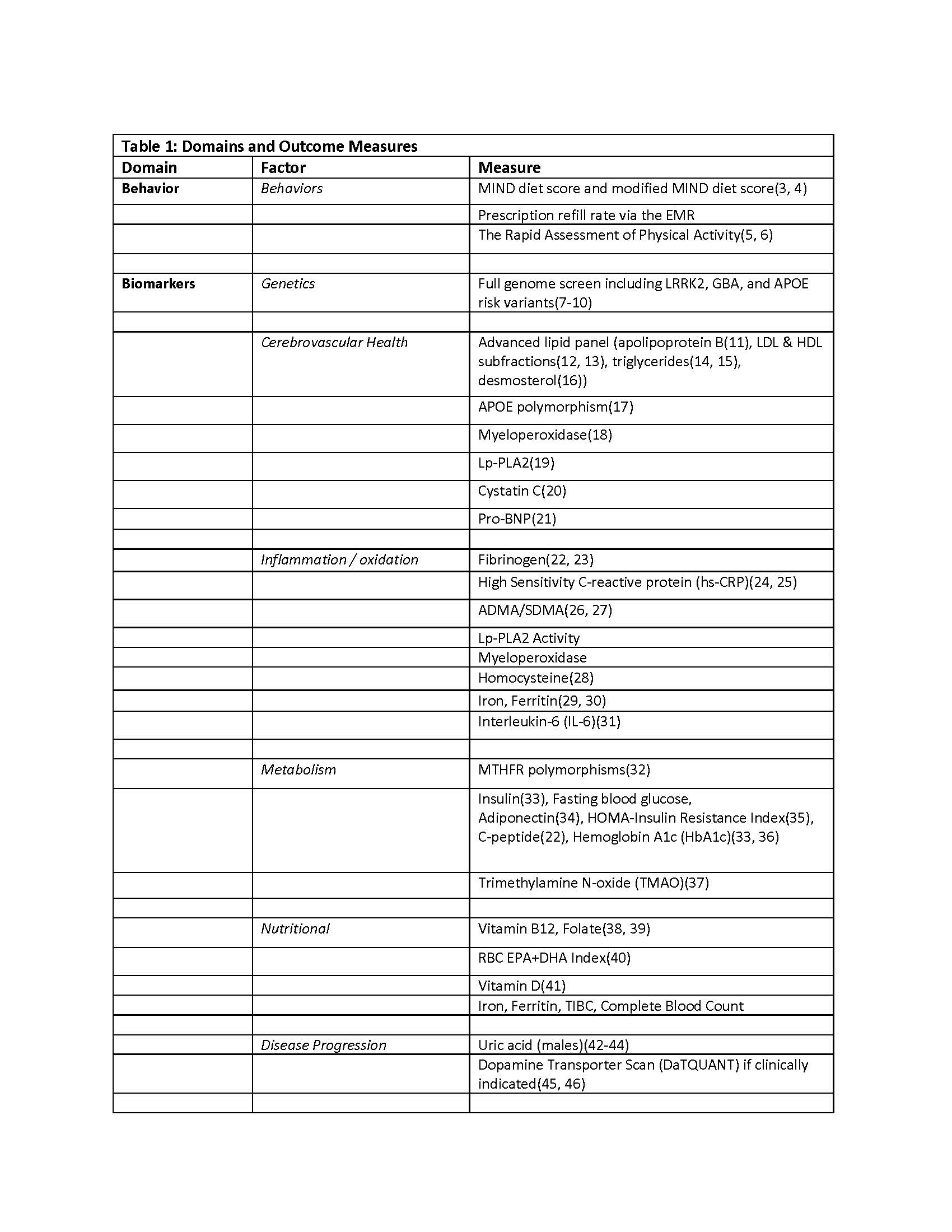
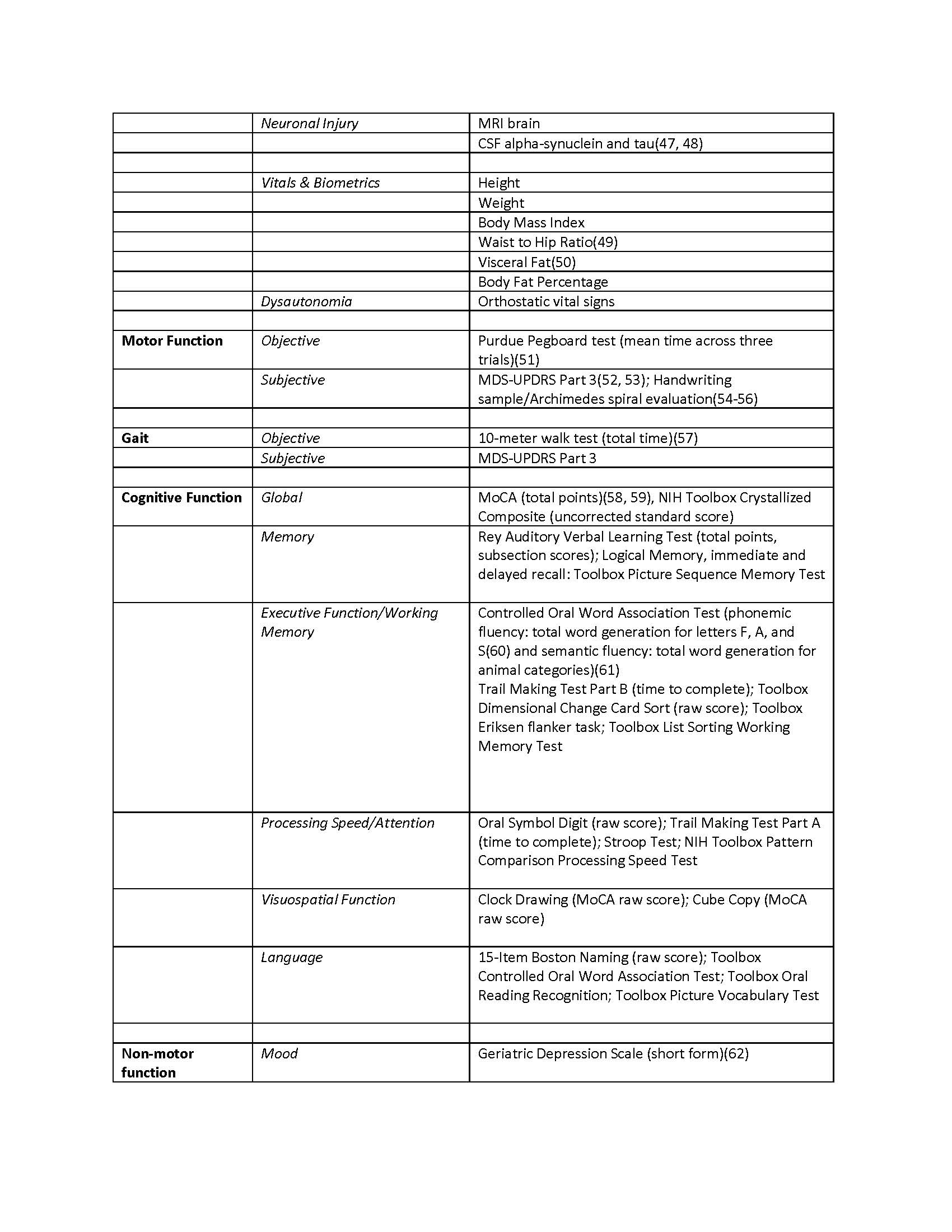
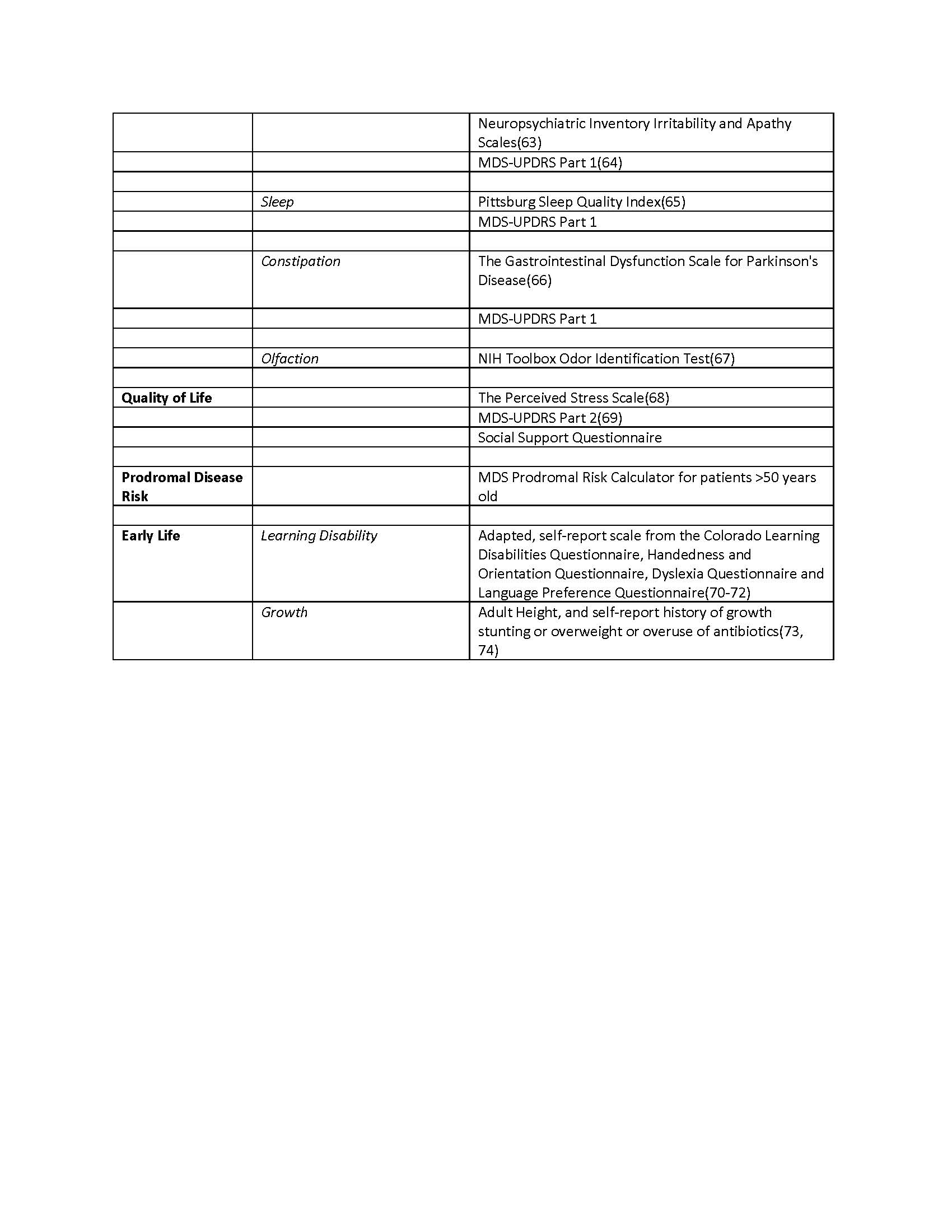
Results: Enrollment into LoPP-PD is ongoing. The initial cohort is 61% male, mean (SD) age was 54.9 (14.8) years, and 43% meet the criteria for highly probable prodromal PD. Table 1 lists current risk profile domains and validated and exploratory outcome measures.
Conclusion: With increasing prevalence, evidence-based personalized interventions to delay PD in those at higher risk is urgently needed. LoPP-PD will provide clinical evidence and provide rationale for personalized interventions to prevent the onset of motor, cognitive, and other nonmotor symptoms of PD.
References: References
1. Isaacson RS, Hristov H, Saif N, Hackett K, Hendrix S, Melendez J, et al. Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimers Dement. 2019;15(12):1588-602.
2. Lee SH, Park SM, Yeo SS, Kwon O, Lee MK, Yoo H, et al. Parkinson’s Disease Subtyping Using Clinical Features and Biomarkers: Literature Review and Preliminary Study of Subtype Clustering. Diagnostics (Basel). 2022;12(1).
3. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015-22.
4. Agarwal P, Wang Y, Buchman AS, Holland TM, Bennett DA, Morris MC. MIND Diet Associated with Reduced Incidence and Delayed Progression of ParkinsonismA in Old Age. J Nutr Health Aging. 2018;22(10):1211-5.
5. Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118.
6. Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PloS one. 2017;12(2):e0170547-e.
7. Li YJ, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, et al. Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology. 2004;62(11):2005-9.
8. Parsian A, Racette B, Goldsmith LJ, Perlmutter JS. Parkinson’s disease and apolipoprotein E: possible association with dementia but not age at onset. Genomics. 2002;79(3):458-61.
9. Tunold J-A, Geut H, Rozemuller JMA, Henriksen SP, Toft M, van de Berg WDJ, et al. APOE and MAPT Are Associated With Dementia in Neuropathologically Confirmed Parkinson’s Disease. Front Neurol. 2021;12.
10. Fyfe I. APOE*ε4 promotes synucleinopathy. Nature Reviews Neurology. 2020;16(4):185-.
11. Gonzalez-Escamilla G, Atienza M, Garcia-Solis D, Cantero JL. Cerebral and blood correlates of reduced functional connectivity in mild cognitive impairment. Brain Struct Funct. 2016;221(1):631-45.
12. Cagnin A, Zambon A, Zarantonello G, Vianello D, Marchiori M, Mercurio D, et al. Serum lipoprotein profile and APOE genotype in Alzheimer’s disease. J Neural Transm Suppl. 2007(72):175-9.
13. Hottman DA, Chernick D, Cheng S, Wang Z, Li L. HDL and cognition in neurodegenerative disorders. Neurobiol Dis. 2014;72 Pt A:22-36.
14. Fang F, Zhan Y, Hammar N, Shen X, Wirdefeldt K, Walldius G, et al. Lipids, Apolipoproteins, and the Risk of Parkinson Disease. Circulation Research. 2019;125(6):643-52.
15. Fu X, Wang Y, He X, Li H, Liu H, Zhang X. A systematic review and meta-analysis of serum cholesterol and triglyceride levels in patients with Parkinson’s disease. Lipids Health Dis. 2020;19(1):97-.
16. Sato Y, Suzuki I, Nakamura T, Bernier F, Aoshima K, Oda Y. Identification of a new plasma biomarker of Alzheimer’s disease using metabolomics technology. J Lipid Res. 2012;53(3):567-76.
17. Hostage CA, Choudhury KR, Murali Doraiswamy P, Petrella JR. Mapping the effect of the apolipoprotein E genotype on 4-year atrophy rates in an Alzheimer disease-related brain network. Radiology. 2014;271(1):211-9.
18. Tzikas S, Schlak D, Sopova K, Gatsiou A, Stakos D, Stamatelopoulos K, et al. Increased myeloperoxidase plasma levels in patients with Alzheimer’s disease. J Alzheimers Dis. 2014;39(3):557-64.
19. Fitzpatrick AL, Irizarry MC, Cushman M, Jenny NS, Chi GC, Koro C. Lipoprotein-associated phospholipase A2 and risk of dementia in the Cardiovascular Health Study. Atherosclerosis. 2014;235(2):384-91.
20. Zhong XM, Hou L, Luo XN, Shi HS, Hu GY, He HB, et al. Alterations of CSF cystatin C levels and their correlations with CSF Αβ40 and Αβ42 levels in patients with Alzheimer’s disease, dementia with lewy bodies and the atrophic form of general paresis. PLoS One. 2013;8(1):e55328.
21. Tynkkynen J, Laatikainen T, Salomaa V, Havulinna AS, Blankenberg S, Zeller T, et al. NT-proBNP and the risk of dementia: a prospective cohort study with 14 years of follow-up. J Alzheimers Dis. 2015;44(3):1007-13.
22. Kiddle SJ, Thambisetty M, Simmons A, Riddoch-Contreras J, Hye A, Westman E, et al. Plasma Based Markers of [11C] PiB-PET Brain Amyloid Burden. PLOS ONE. 2012;7(9):e44260.
23. Ahn HJ, Glickman JF, Poon KL, Zamolodchikov D, Jno-Charles OC, Norris EH, et al. A novel Aβ-fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer’s disease mice. J Exp Med. 2014;211(6):1049-62.
24. Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease–associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183-9.
25. Qiu X, Xiao Y, Wu J, Gan L, Huang Y, Wang J. C-Reactive Protein and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Neurol. 2019;10.
26. Kirbas S, Kirbas A, Tufekci A, Cumhur Cure M, Cakmak S, Yazici T, et al. Serum levels of homocysteine, asymmetric dimethylarginine and nitric oxide in patients with Parkinson’s disease. Acta Clin Belg. 2016;71(2):71-5.
27. Sankowski B, Księżarczyk K, Raćkowska E, Szlufik S, Koziorowski D, Giebułtowicz J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin Chim Acta. 2020;501:165-73.
28. Chen H, Liu S, Ji L, Wu T, Ma F, Ji Y, et al. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: a case-control study. Curr Alzheimer Res. 2015;12(1):88-94.
29. Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, et al. Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol. 1994;36(6):876-81.
30. Wu L, Gerber O. Association of Serum Ferritin with Parkinsonian Disorders (P04.162). Neurology. 2013;80(7 Supplement):P04.162.
31. Green HF, Khosousi S, Svenningsson P. Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease. Journal of Parkinson’s Disease. 2019;9:705-9.
32. Peng Q, Lao X, Huang X, Qin X, Li S, Zeng Z. The MTHFR C677T polymorphism contributes to increased risk of Alzheimer’s disease: evidence based on 40 case-control studies. Neurosci Lett. 2015;586:36-42.
33. Hogg E, Athreya K, Basile C, Tan EE, Kaminski J, Tagliati M. High Prevalence of Undiagnosed Insulin Resistance in Non-Diabetic Subjects with Parkinson’s Disease. J Parkinsons Dis. 2018;8(2):259-65.
34. Li W, Yu Z, Hou D, Zhou L, Deng Y, Tian M, et al. Relationship between Adiponectin Gene Polymorphisms and Late-Onset Alzheimer’s Disease. PLoS One. 2015;10(4):e0125186.
35. Luciano R, Barraco GM, Muraca M, Ottino S, Spreghini MR, Sforza RW, et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. 2015;135(6):1074-81.
36. Zittel S, Uyar M, Lezius S, Gerloff C, Choe C-u. HbA1c and Motor Outcome in Parkinson’s Disease in the Mark-PD Study. Movement Disorders. 2021;36(8):1991-2.
37. Chung SJ, Rim JH, Ji D, Lee S, Yoo HS, Jung JH, et al. Gut microbiota-derived metabolite trimethylamine N-oxide as a biomarker in early Parkinson’s disease. Nutrition. 2021;83:111090.
38. Schaffner A, Li X, Gomez-Llorente Y, Leandrou E, Memou A, Clemente N, et al. Vitamin B(12) modulates Parkinson’s disease LRRK2 kinase activity through allosteric regulation and confers neuroprotection. Cell Res. 2019;29(4):313-29.
39. McCarter SJ, Stang C, Turcano P, Mielke MM, Ali F, Bower JH, et al. Higher vitamin B12 level at Parkinson’s disease diagnosis is associated with lower risk of future dementia. Parkinsonism Relat Disord. 2020;73:19-22.
40. Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, et al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78(9):658-64.
41. Berti V, Murray J, Davies M, Spector N, Tsui WH, Li Y, et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging. 2015;19(4):413-23.
42. de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58(5):797-800.
43. Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166(5):561-7.
44. Gao X, O’Reilly É J, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. 2016;86(6):520-6.
45. Ikeda K, Ebina J, Kawabe K, Iwasaki Y. Dopamine Transporter Imaging in Parkinson Disease: Progressive Changes and Therapeutic Modification after Anti-parkinsonian Medications. Intern Med. 2019;58(12):1665-72.
46. Brogley JE. DaTQUANT: The Future of Diagnosing Parkinson Disease. Journal of Nuclear Medicine Technology. 2019;47(1):21.
47. Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M, et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurology. 2021;78(1):30-40.
48. Vieira SRL, Toffoli M, Campbell P, Schapira AHV. Biofluid Biomarkers in Parkinson’s Disease: Clarity Amid Controversy. Mov Disord. 2020;35(7):1128-33.
49. Kim HJ, Kim C, Jeon S, Kang M, Kim YJ, Lee JM, et al. Association of Body Fat Percentage and Waist-hip Ratio With Brain Cortical Thickness: A Study Among 1777 Cognitively Normal Subjects. Alzheimer Dis Assoc Disord. 2015;29(4):279-86.
50. Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, Massaro JM, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68(2):136-44.
51. Proud EL, Miller KJ, Bilney B, Morris ME, McGinley JL. Construct validity of the 9-Hole Peg Test and Purdue Pegboard Test in people with mild to moderately severe Parkinson’s disease. Physiotherapy. 2020;107:202-8.
52. Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson’s disease rating scale scores to Movement Disorder Society-unified Parkinson’s disease rating scale scores. Movement Disorders. 2012;27(10):1239-42.
53. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-70.
54. Kamble M, Shrivastava P, Jain M. Digitized spiral drawing classification for Parkinson’s disease diagnosis. Measurement: Sensors. 2021;16:100047.
55. Zham P, Kumar DK, Dabnichki P, Poosapadi Arjunan S, Raghav S. Distinguishing Different Stages of Parkinson’s Disease Using Composite Index of Speed and Pen-Pressure of Sketching a Spiral. Front Neurol. 2017;8.
56. Zham P, Arjunan SP, Raghav S, Kumar DK. Efficacy of Guided Spiral Drawing in the Classification of Parkinson’s Disease. IEEE J Biomed Health Inform. 2018;22(5):1648-52.
57. Lindholm B, Nilsson MH, Hansson O, Hagell P. The clinical significance of 10-m walk test standardizations in Parkinson’s disease. J Neurol. 2018;265(8):1829-35.
58. Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738-45.
59. Fengler S, Kessler J, Timmermann L, Zapf A, Elben S, Wojtecki L, et al. Screening for Cognitive Impairment in Parkinson’s Disease: Improving the Diagnostic Utility of the MoCA through Subtest Weighting. PLoS One. 2016;11(7):e0159318.
60. Jones S, Laukka EJ, Bäckman L. Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia. Cortex. 2006;42(3):347-55.
61. Mueller KD, Koscik RL, LaRue A, Clark LR, Hermann B, Johnson SC, et al. Verbal Fluency and Early Memory Decline: Results from the Wisconsin Registry for Alzheimer’s Prevention. Arch Clin Neuropsychol. 2015;30(5):448-57.
62. Ertan FS, Ertan T, Kiziltan G, Uyguçgil H. Reliability and validity of the Geriatric Depression Scale in depression in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76(10):1445-7.
63. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233-9.
64. Gallagher DA, Goetz CG, Stebbins G, Lees AJ, Schrag A. Validation of the MDS-UPDRS Part I for nonmotor symptoms in Parkinson’s disease. Mov Disord. 2012;27(1):79-83.
65. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213.
66. Camacho M, Greenland JC, Williams-Gray CH. The Gastrointestinal Dysfunction Scale for Parkinson’s Disease. Movement Disorders. 2021;36(10):2358-66.
67. Dalton P, Doty RL, Murphy C, Frank R, Hoffman HJ, Maute C, et al. Olfactory assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S32.
68. van der Heide A, Speckens AEM, Meinders MJ, Rosenthal LS, Bloem BR, Helmich RC. Stress and mindfulness in Parkinson’s disease – a survey in 5000 patients. npj Parkinson’s Disease. 2021;7(1):7.
69. Ramsay N, Macleod AD, Alves G, Camacho M, Forsgren L, Lawson RA, et al. Validation of a UPDRS-/MDS-UPDRS-based definition of functional dependency for Parkinson’s disease. Parkinsonism Relat Disord. 2020;76:49-53.
70. Patrick KE, McCurdy MD, Chute DL, Mahone EM, Zabel TA, Jacobson LA. Clinical Utility of the Colorado Learning Difficulties Questionnaire. Pediatrics. 2013;132(5):e1257-e64.
71. Tamboer P, Vorst HC, Oort FJ. Identifying dyslexia in adults: an iterative method using the predictive value of item scores and self-report questions. Ann Dyslexia. 2014;64(1):34-56.
72. Nergård-Nilssen T, Hulme C. Developmental dyslexia in adults: behavioural manifestations and cognitive correlates. Dyslexia. 2014;20(3):191-207.
73. Mertsalmi TH, Pekkonen E, Scheperjans F. Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov Disord. 2020;35(3):431-42.
74. Russ TC, Kivimäki M, Starr JM, Stamatakis E, Batty GD. Height in relation to dementia death: individual participant meta-analysis of 18 UK prospective cohort studies. Br J Psychiatry. 2014;205(5):348-54.
To cite this abstract in AMA style:
K. Niotis, K. Akiyoshi, R. Isaacson, S. Isaacson. Longitudinal Program to Prevent PD (LoPP-PD): Multimodal Risk Stratification Informs Personalized Intervention [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/longitudinal-program-to-prevent-pd-lopp-pd-multimodal-risk-stratification-informs-personalized-intervention/. Accessed April 2, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/longitudinal-program-to-prevent-pd-lopp-pd-multimodal-risk-stratification-informs-personalized-intervention/