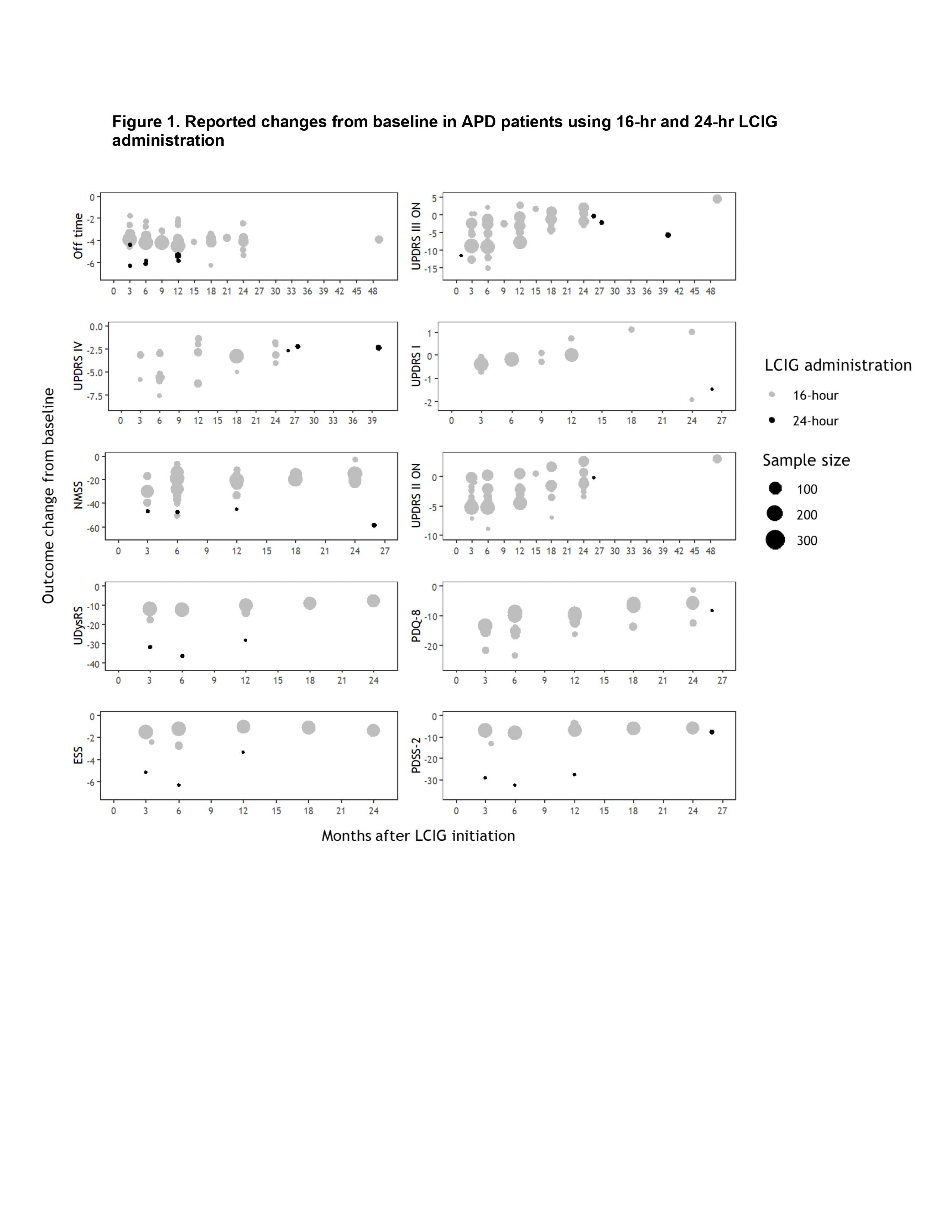
Objective: To evaluate any existing differences between the reported outcomes in patients with advanced Parkinson’s disease (APD) using continuous 16-hour (hr) infusions of levodopa-carbidopa intestinal gel (LCIG) versus 24-hr infusions.
Background: PD is a chronic and progressive disorder primarily associated with the degeneration of brain dopamine neurons and depletion of striatal dopamine. LCIG is a stable gel suspension suitable for continuous delivery through percutaneous gastrojejunostomy via a portable pump. Treatment is usually administered during the patient’s awake period. If medically justified, LCIG may be administered for up to 24 hrs.
Method: Published outcome data for patients using 24-hr LCIG were obtained from 7 publications identified from a targeted search of the literature. Reported clinical outcomes were qualitatively compared to those reported within studies of 16-hr LCIG identified in a previously published review to identify any differences or trends between the two administration schedules. Sample sizes of the 24-hr studies were small, ranging from 3 to 34, which prohibited any reliable quantitative comparison of the reported 24-hr and 16-hr data. Outcomes of interest included change from baseline in off time, motor symptoms (UPDRS III and IV), non-motor symptoms (UPDRS I, NMSS), activities of daily living (UPDRS II), dyskinesia (UDysRS), quality of life (PDQ-8), and sleep (ESS and PDSS-2).
Results: Comparing the characteristics of patients using 24-hr LCIG to those using 16-hr administration revealed no systematic differences. Outcomes with 24-hr administration showed greater decreases from baseline in off time than 16-hr (Figure 1). Similarly, greater decreases from baseline were reported in the 24-hr studies of UPDRS I, NMSS, UDysRS, ESS, and PDSS-2. Comparable changes from baseline between the two administration strategies were observed for UPDRS II ON, UPDRS III ON, UPDRS IV, and PDQ-8. Safety was consistent with the established LCIG profile.
Conclusion: The available 24-hr administration data showed comparable or better reductions in several clinical and quality of life outcomes than the 16-hr data. When appropriate, continuous daily (24 hr) administration could confer additional benefit to APD patients receiving LCIG.
To cite this abstract in AMA style:
N. Kovács, M. Simu, O. Sanchez Soliño, J. Parra, J. Alcazar, S. Snedecor, V. Fung. Reported Clinical and Quality-of-Life Outcomes With 24-Hour Levodopa-Carbidopa Intestinal Gel Administration Compared With 16-Hour Administration [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/reported-clinical-and-quality-of-life-outcomes-with-24-hour-levodopa-carbidopa-intestinal-gel-administration-compared-with-16-hour-administration/. Accessed December 29, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/reported-clinical-and-quality-of-life-outcomes-with-24-hour-levodopa-carbidopa-intestinal-gel-administration-compared-with-16-hour-administration/