Category: Parkinson's Disease: Neurophysiology
Objective: Our objective is to assess how well short-latency afferent inhibition (SAI) represents cortical cholinergic system integrity in patients with Parkinson’s disease (PD), using cholinergic PET imaging as a reference.
Background: It has been shown that degeneration of the cholinergic system in PD is related to both motor and non-motor symptoms, including cognitive impairment, REM-sleep behavioral disorder, visual hallucinations, hyposmia, postural instability and gait disorder [1][2]. SAI, which is assessed using transcranial magnetic stimulation (TMS) paired with peripheral electrical stimulation, is often viewed as a cholinergic marker in PD, but this has never been confirmed with neuroimaging. Based on the working model of SAI [3], we expect the strongest relation between SAI and cholinergic imaging in the primary motor cortex.
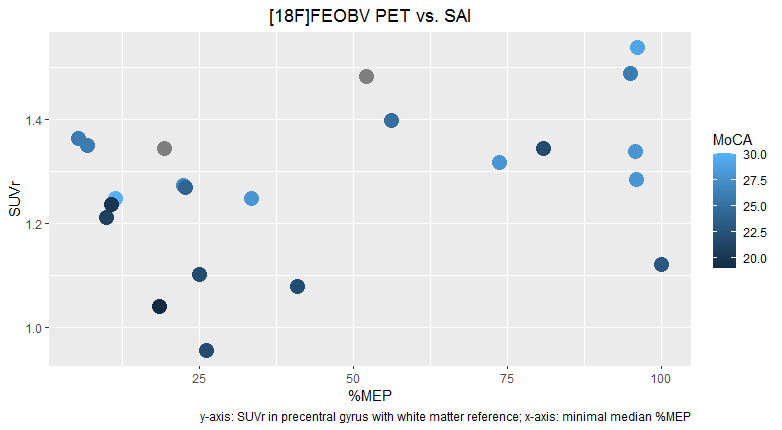
Method: We performed both SAI and [18F]FEOBV PET imaging in PD patients. SAI was performed on the most affected side, using 5 different interstimulus intervals (ISIs). We applied 15 stimulations per ISI and 15 unconditioned stimulations (i.e. single pulse TMS to the motor cortex, without peripheral stimulation), in a random order. For each patient, the median %MEP (median motor evoked potential as a percentage of the median MEP of the unconditioned stimulations) was calculated for all intervals. The smallest median %MEP, i.e. the median %MEP of the ISI that showed the strongest inhibition, was used as a main outcome. For the [18F]FEOBV PET imaging, the standardized uptake value ratio (SUVr) was calculated for the precentral gyrus of the most affected hemisphere, with supratentorial white matter as a reference.
Results: Results from the first 22 subjects, 4 female, are presented. Mean age was 66 years (SD: 8.3) and mean MoCA (Montreal Cognitive Assessment) score was 24.8/30 (SD: 3.3). Mean SUVr was 1.27 (SD: 0.15) and mean %MEP 45.4 (SD: 34.8). A scatter plot is presented in Figure 1. A linear regression model didn’t fit the data well (R2=0.14, p=0.10).
[figure 1]
Conclusion: We expected a negative relation between SUVr and mean %MEP, which appears to be reflected in the preliminary results for minimal median %MEP<50 (R2=0.28, p=0.06). However, for %MEP>50, the relation is less clear, which raises questions about the usefulness of SAI as a cholinergic marker. Inclusion is ongoing and a healthy control group will be added for comparison.
References: [1] Müller, M., & Bohnen, N. (2013). Cholinergic Dysfunction in Parkinson’s Disease. Curr Neurol Neurosci Rep, 13, 337. https://doi.org/10.1007/s11910-013-0377-9
[2] Liu, A. K. L., Chang, R. C. C., Pearce, R. K. B., & Gentleman, S. M. (2015). Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathologica, 129(4), 527–540. https://doi.org/10.1007/s00401-015-1392-5
[3] Turco, C. V, El-sayes, J., Savoie, M. J., Fassett, H. J., Locke, M. B., & Nelson, A. J. (2018). Brain Stimulation Short- and long-latency afferent inhibition ; uses , mechanisms and influencing factors. Brain Stimulation, 11(1), 59–74. https://doi.org/10.1016/j.brs.2017.09.009
To cite this abstract in AMA style:
E. D'Angremont, I. Zijdewind, S. Vander Zee, E. de Vries, T. van Laar, I. Sommer. Short-latency afferent inhibition for the assessment of cholinergic degeneration in Parkinson’s disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/short-latency-afferent-inhibition-for-the-assessment-of-cholinergic-degeneration-in-parkinsons-disease/. Accessed December 13, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/short-latency-afferent-inhibition-for-the-assessment-of-cholinergic-degeneration-in-parkinsons-disease/