Category: Rare Genetic and Metabolic Diseases
Objective: Evaluate the safety and efficacy of a conservative mode of iron chelation with deferiprone 30 mg/kg/day in neuroferritinopathy to limit iron-related neurodegeneration and associated clinical disability.
Background: Neuroferritinopathy is a rare inherited neurodegeneration with brain iron accumulation (NBIA) characterized by iron overload in various parts of the brain, particularly the basal ganglia and cerebellar nuclei, resulting in movement disorders and/or progressive cerebellar signs in children or young adults. [1] No treatment is currently available for neuroferritinopathy. A recent trial of deferiprone in pantothenate kinase-associated degeneration type 2 showed that deferiprone was well tolerated, achieved its intended goal (reduction of iron in the basal ganglia), and appeared to slow disease progression somewhat at 18 months. [2]
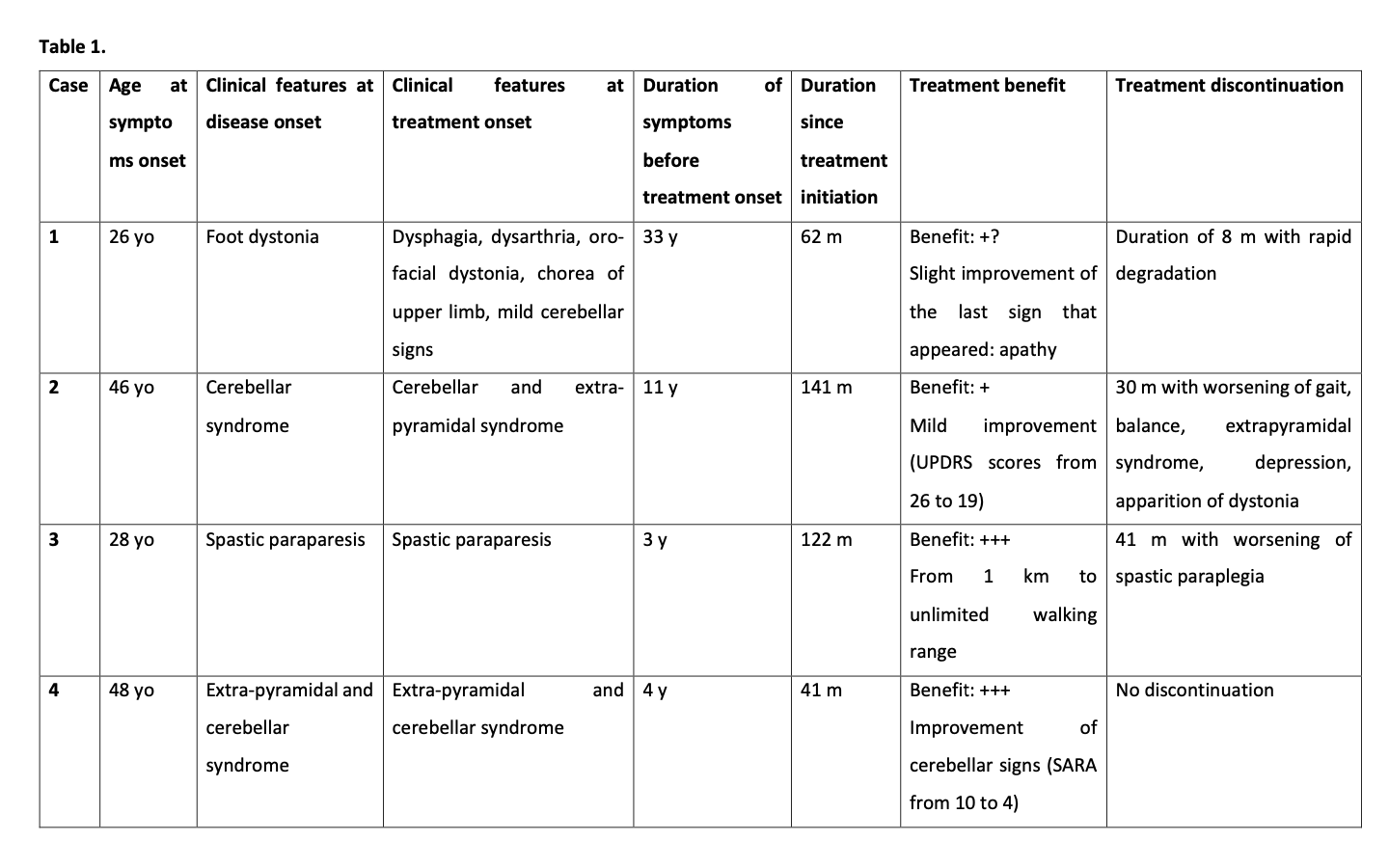
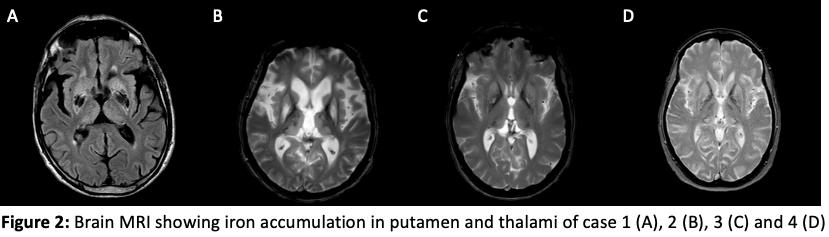
Method: Four patients from the same family with genetically confirmed diagnostic of neuroferritinopathy (458dupA mutation of FTL1) was placed under deferiprone 30 mg/kg/j at different stage of progression. They underwent clinical and MRI follow-up to assess efficacity of the treatment on symptoms and brain iron overload in basal ganglia. They were also biologically monitored to control risk of adverse event, especially neutropenia.
Results: The four cases experienced different levels of therapeutic response depending on the age of the disease and the severity of the cerebral iron overload and the clinical picture, ranging from no or little subjective improvement of the symptoms in the severe and advanced cases (cases 1 and 2) to frank clinical improvement followed by maintenance of the benefit without any progression for several years in the two beginner cases (cases 3 and 4) with an age of signs of less than 4 years.
Due to agranulocytosis, pregnancy or patient’s wish, several treatment discontinuations were performed, and all resulted in a frank and faster worsening of symptoms. [table1] In case 3, the mild clinical disability was maintained without any progression for more than 11 years. In case 4, the symptoms improved dramatically after a few months of treatment. None of them suffered from anemia.
Conclusion: These initial data suggest that conservative iron chelation with deferiprone may be an effective and safe way to treat neuroferritinopathy. It seems that the benefit can be very great in case of very early treatment at the time of the diagnosis with a delay since the first symptoms of less than 5 years.
References: 1. Schipper HM. Neurodegeneration with brain iron accumulation – clinical syndromes and neuroimaging. Biochim Biophys Acta. mars 2012;1822(3):350‑60.
2. Klopstock T, Tricta F, Neumayr L, Karin I, Zorzi G, Fradette C, et al. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: a randomised, double-blind, controlled trial and an open-label extension study. The Lancet Neurology. 1 juill 2019;18(7):631‑42.
To cite this abstract in AMA style:
F. Marchand, C. Moreau, G. Kuchcinski, V. Huin, L. Defebvre, D. Devos. Conservative iron chelation for Neuroferritinopathy [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/conservative-iron-chelation-for-neuroferritinopathy/. Accessed March 31, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/conservative-iron-chelation-for-neuroferritinopathy/