Category: Parkinson's Disease: Neuroimaging
Objective: To identity the effect of exercise on brain metabolism and energetics in PD using PET/MR imaging.
Background: Aerobic exercise slows PD progression as reflected clinically [1]. Mitochondrial dysfunction may play a role in the onset and progression of PD, and aerobic exercise may positively modulate mitochondrial dynamics [2]. PET/MR imaging can be used to obtain simultaneously cerebral metabolic rates of oxygen (CMRO2) and glucose (CMRglu); estimates of mitochondrial function can be inferred through the oxygen-to-glucose ratio.
Method: An ongoing study is performing dual-calibrated BOLD MRI and FDG PET imaging to obtain CMRO2 and CMRglu in PD habitual exercisers (PD-HE, n=4) and PD non-exercisers (PD-NE) before (n=6) and after (n=4) six-month stationary cycling. Constrained principal component analysis (CPCA) [3] is used to identify disease- and exercise-related spatial patterns of regional CMRO2 and CMRglu. CPCA first regresses Z, the spatially-normalized imaging measures for each subject, onto clinical variables, X, related to disease (disease duration (DD), UPDRS-III) and aerobic fitness (VO2 max), i.e. Z ≈ XC, where C are the regression coefficients. Standard PCA is then run on XC to obtain spatial patterns rank-ordered by their explanation of the variance-of-interest contained in XC.
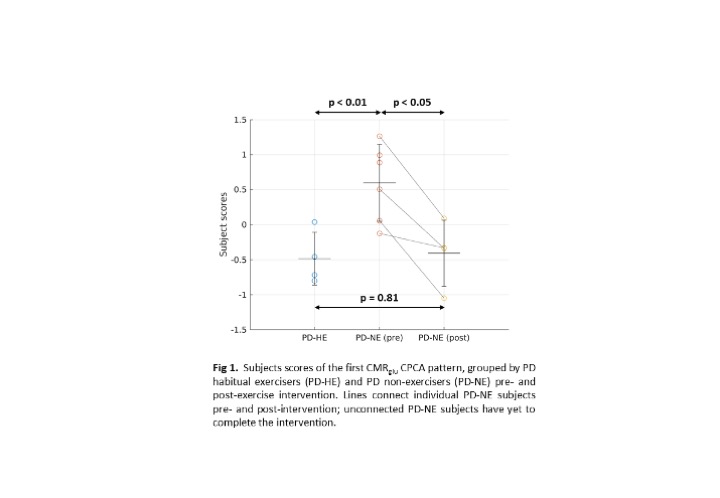
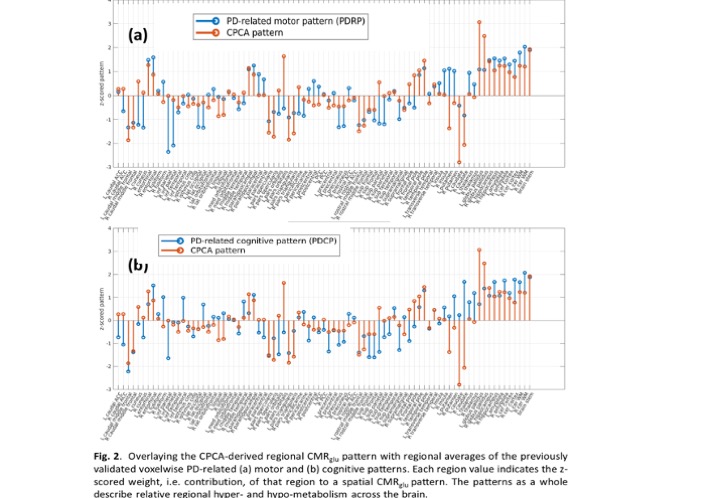
Results: Subject scores of the first CMRglu CPCA pattern are significantly higher in the PD-NE pre-intervention compared to PD-HE (p<0.01, [figure 1]) and PD-NE post intervention (p<0.05). No significant difference was found between PD-HE and PD-NE after intervention. This pattern has significant overlap with the PD disease-related and PD cognition-related [4] patterns ([figure 2], PDRP: ρ=0.57; PDCP: ρ=0.51), known to correlate with disease severity. Subject scores correlate positively with DD (R2=0.36, p<0.05), negatively with VO2 max (R2=0.70, p<0.001), and have no correlation with UPDRS-III, indicating most of the CPCA pattern variance is governed by exercise-induced changes. A similar initial CMRO2 analysis did not yield group differences.
Conclusion: A pattern of glucose metabolism positively related to DD and negatively related to aerobic fitness was found. Group differences of pattern expression between a small cohort of PD non-exercisers before and after aerobic exercise suggests causality between exercise and disease progression. More subjects will be added as they complete the intervention. Joint CMRglu and CMRO2 pattern analysis will follow.
References: [1] Tsukita K, Sakamaki-Tsukita H, Takahashi R. Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With Early Parkinson Disease. Neurology. 2022 Feb 22;98(8):e859-e871. doi: 10.1212/WNL.0000000000013218. Epub 2022 Jan 12. PMID: 35022304; PMCID: PMC8883509.
[2] Chuang CS, Chang JC, Cheng FC, Liu KH, Su HL, Liu CS. Modulation of mitochondrial dynamics by treadmill training to improve gait and mitochondrial deficiency in a rat model of Parkinson’s disease. Life Sci. 2017 Dec 15;191:236-244. doi: 10.1016/j.lfs.2017.10.003. Epub 2017 Oct 3. PMID: 28986095.
[3] Takane Y & Shibayama, T. Principal component analysis with external information on both subjects and variables. Psychometrika. Mar 1991; 56(1):97–120. https://doi.org/10.1007/BF02294589.
[4] Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009 Oct;32(10):548-57. doi: 10.1016/j.tins.2009.06.003. Epub 2009 Sep 16. PMID: 19765835; PMCID: PMC2782537.
To cite this abstract in AMA style:
C. Bevington, R. Jayde Williams, B. Pike, J. Mckenzie, J. Zhang, W. Luh, AJ. Stoessl, V. Sossi. Effect of exercise on brain metabolism and energetics in PD [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/effect-of-exercise-on-brain-metabolism-and-energetics-in-pd/. Accessed March 31, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/effect-of-exercise-on-brain-metabolism-and-energetics-in-pd/