Category: Parkinsonism, Atypical: MSA
Objective: The putamen is a difficult structure to segment accurately, and automated methods require manual editing to improve segmentation accuracy. Our aim was to test the sensitivity of a novel, automated AI algorithm in measuring putamen volume over time in MSA.
Background: We developed a novel AI platform using deep learning to enable sensitive and reliable automated measurement of volume and volume change. We tested the sensitivity of the platform in MSA using 3D T1-weighted (T1W) images from the M-STAR clinical trial.
Method: T1W scans were collected from 47 sites at screening and week 48 or early termination visits. In total we analyzed 329 baseline scans and 257 follow-up scans from randomized participants with both the parkinsonian (MSA-P) and cerebellar MSA subtype (MSA-C). There were no healthy controls and we did not differentiate between placebo and treatment groups, because patients were randomized by MSA subtype and diagnostic category.
Our automated AI-based method measures regional volume from segmentations based on convolutional neural networks1–3, and volume change by integrating the log jacobian determinant of deformation fields4 within regional masks. For this work, we generated automated segmentations at baseline of the left and right putamen. To test the sensitivity of the developed platform to disease subtype and progression, we used generalised linear models (GLM) testing for the effect of MSA subtype (MSA-C vs MSA-P), total UMSARS, region (left and right putamen) and their interactions on baseline volume and volume change. Volume was adjusted for total intracranial volume (TIV) and volume change was annualized. The models were adjusted for age, sex and site.
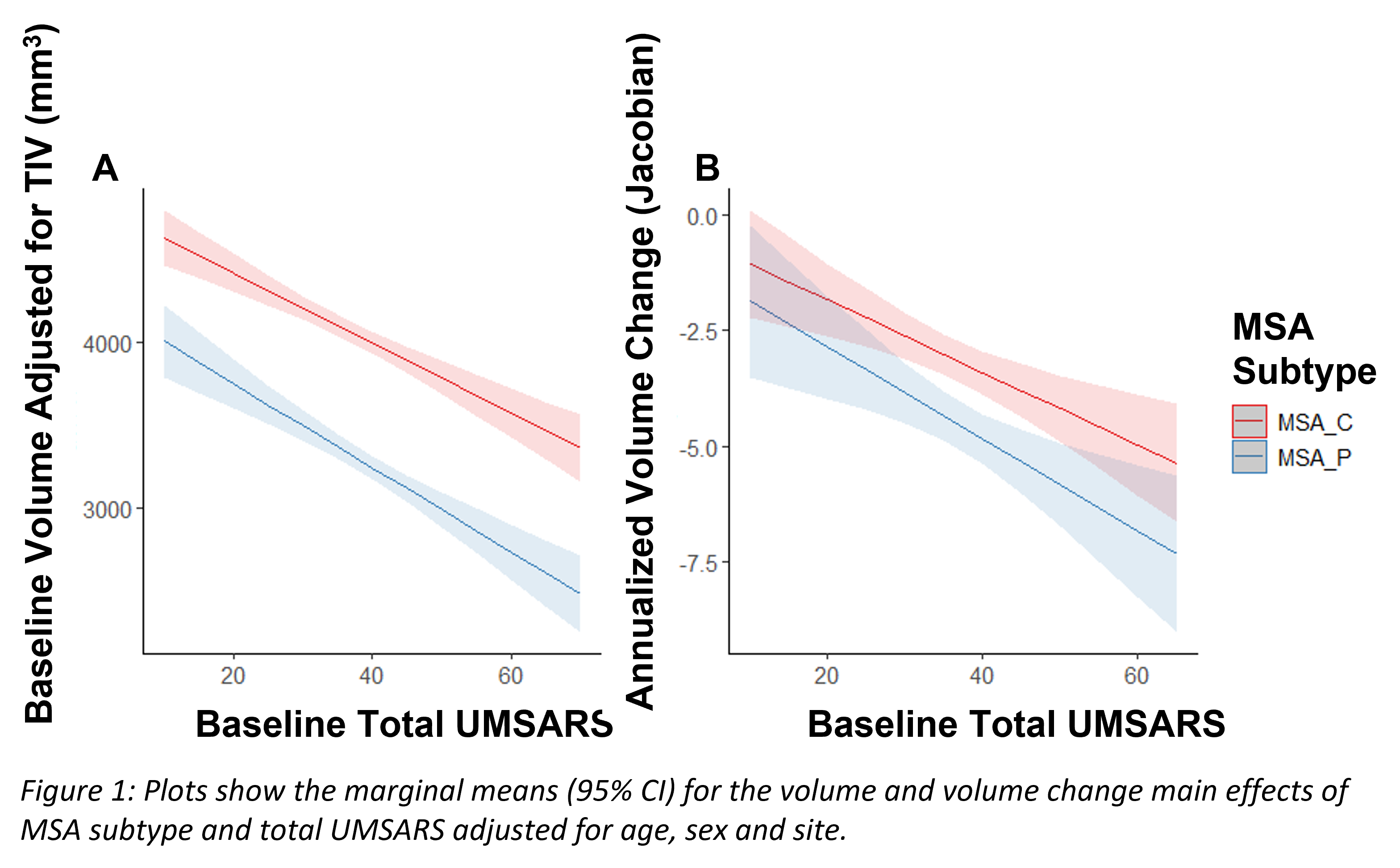
Results: For both TIV adjusted baseline volume and annualized volume change there was a significant main effect of MSA subtype and UMSARS (all p<0.001), there was no significant difference between left and right putamen or any interactions. Patients with higher UMSARS score (irrespective of subtype) had smaller putamen volume than those with lower score, and MSA-P patients had smaller putamen volume than MSA-C patients (Figure 1A). Similarly for annualized volume change, putamen volume change was greater for patients with higher UMSARS score, and for MSA-P patients than MSA-C patients (Figure 1B).
Conclusion: Conclusion:We have shown that our fully-automated method provides measures of volume and volume change sensitive to disease subtype and progression in MSA.
References: 1. Weatheritt J, Joules R, Wolz R, Rueckert D. Fully Automatic AI Segmentation of Subcortical Regions. Neurotherapeutics. 2020;17(SUPPL 1):21-21.
2. Weatheritt J, Rueckert D, Wolz R. Transfer Learning for Brain Segmentation: Pre-task Selection and Data Limitations. In: ; 2020:118-130. doi:10.1007/978-3-030-52791-4_10
3. Weatheritt J, Reinwald M, Brito B, Wolz R. AUTOMATIC SEGMENTATION USING DEEP LEARNING FOR THE HIPPOCAMPUS. In: ; 2021.
4. Reinwald M, Joules R, Papoutsi M, Weatheritt J, Wolz R. Towards a fully automatic AI framework to calculate voxel-wise atrophy in the human brain. Alzheimers Dement. 2021;17(S5):e050068. doi:10.1002/alz.050068
To cite this abstract in AMA style:
M. Papoutsi, J. Weatheritt, M. Reinwald, I. Gidado, R. Joules, H. Kaufmann, I. Qureshi, R. Wolz. An automated AI-based framework for putamen volume measurement in MSA [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/an-automated-ai-based-framework-for-putamen-volume-measurement-in-msa/. Accessed February 28, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/an-automated-ai-based-framework-for-putamen-volume-measurement-in-msa/