Category: Other
Objective: To explore the long-term safety and efficacy of focused ultrasound subthalamotomy (FUS-STN).
Background: Unilateral MRI-guided high-intensity FUS-STN improves motor signs of Parkinson’s disease (PD) [1]
Method: A prospective, open-label study of asymmetrical PD patients who underwent unilateral FUS-STN. All patients were evaluated up to 24-36 months after treatment, with a subgroup reaching 5-years of follow-up. The primary outcome was the difference from baseline to 24-36 months after FUS-STN in the Movement Disorders Society-Unified Rating scale (MDS-UPDRS) motor part (III) for the treated hemibody in the off-medication state. Safety outcomes included all reported adverse events. Secondary outcomes were MDS-UPDRS III score for the treated side on-medication; total MDS-UPDRS III; MDS-UPDRS part IV; medication change; functional disability (MDS-UPDRS-II); and quality of life (PDQ39SI). Patient satisfaction and clinical impression of change (PGIC) were self-assessed.
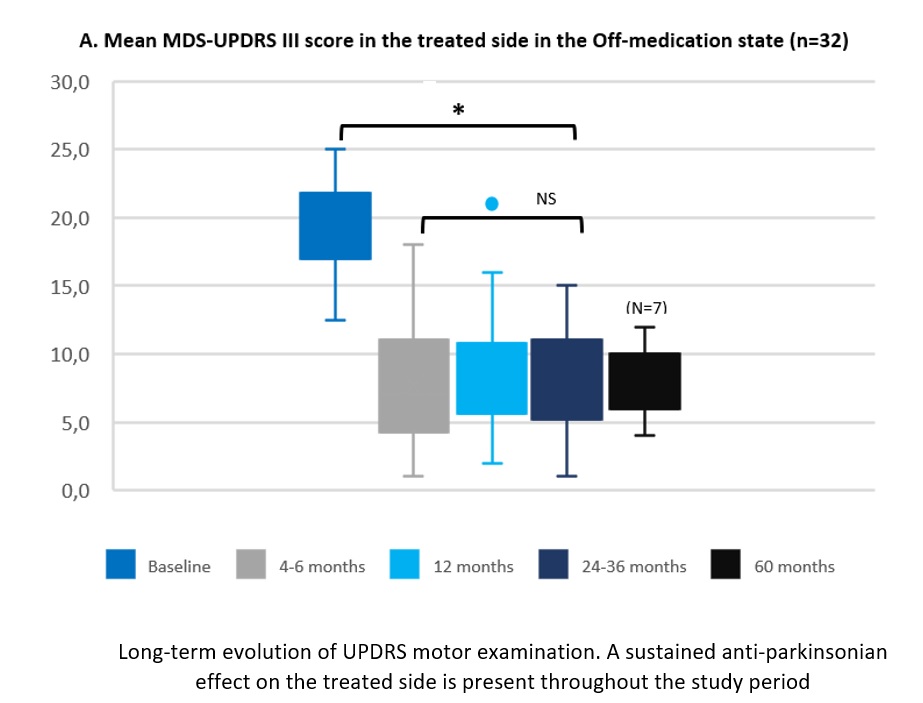
Results: Thirty-two PD patients were evaluated 2-3 years after treatment, and 7 reached 5 years follow-up. The mean (±SD) age at baseline was 56.0±10.1 years, with a mean disease duration of 6.8±2.8 years. The MDS-UPDRS III score for the treated body side off-medication improved by 54.3% from baseline to 2-3 years (19.0±3.2 to 8.6±3.3, p<0.001). All cardinal motor features remained significantly improved compared with baseline. No disabling or delayed adverse events were reported at 2-3 years. One patient in the 5-year subgroup presented disabling peak-of-dose dyskinesia in the treated arm. Total MDS-UPDRS III off-medication score was 26.4% lower at 2-3 years than before treatment (36.3±7.0 vs 26.3±7.0, p<0.001). MDS-UPDRS II, IV, and PDQ39SI scores were stable. The 5-year follow-up subgroup elicited similar results. Most patients self-assessed a better global status at 2-3 years follow-up than before FUS-STN (96.3%) and were satisfied with the treatment (86.2%).
Conclusion: The effect of unilateral FUS-STN in PD is sustained over long-term (3-5 years) evolution without medical management complications and delayed adverse events.
References: [1] Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, Del Álamo M, Shah BB, Hernández-Fernández F, Pineda-Pardo JA, Monje MHG, Fernández-Rodríguez B, Sperling SA, Mata-Marín D, Guida P, Alonso-Frech F, Obeso I, Gasca-Salas C, Vela-Desojo L, Elias WJ, Obeso JA. Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease. N Engl J Med. 2020 Dec 24;383(26):2501-2513. doi: 10.1056/NEJMoa2016311. PMID: 33369354.
To cite this abstract in AMA style:
R. Martínez-Fernández, E. Natera-Villalba, J. Máñez-Miró, R. Rodríguez-Rojas, M. Del Alamo, J. Pineda-Pardo, I. Obeso, F. Hernández-Fernández, C. Gasca-Salas, M. Matarazzo, L. Vela, F. Alonso-Frech, J. Obeso. Long-term efficacy of unilateral high-intensity focused ultrasound subthalamotomy for Parkinson’s Disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/long-term-efficacy-of-unilateral-high-intensity-focused-ultrasound-subthalamotomy-for-parkinsons-disease/. Accessed March 6, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-efficacy-of-unilateral-high-intensity-focused-ultrasound-subthalamotomy-for-parkinsons-disease/