Category: Parkinson’s Disease: Clinical Trials
Objective: Modeling Parkinson’s disease in mice suggests c-Abl activation is required for PD initiation and progression, and therefore inhibition of c-Abl could be a strategy for disease-modification of Parkinson’s (1).
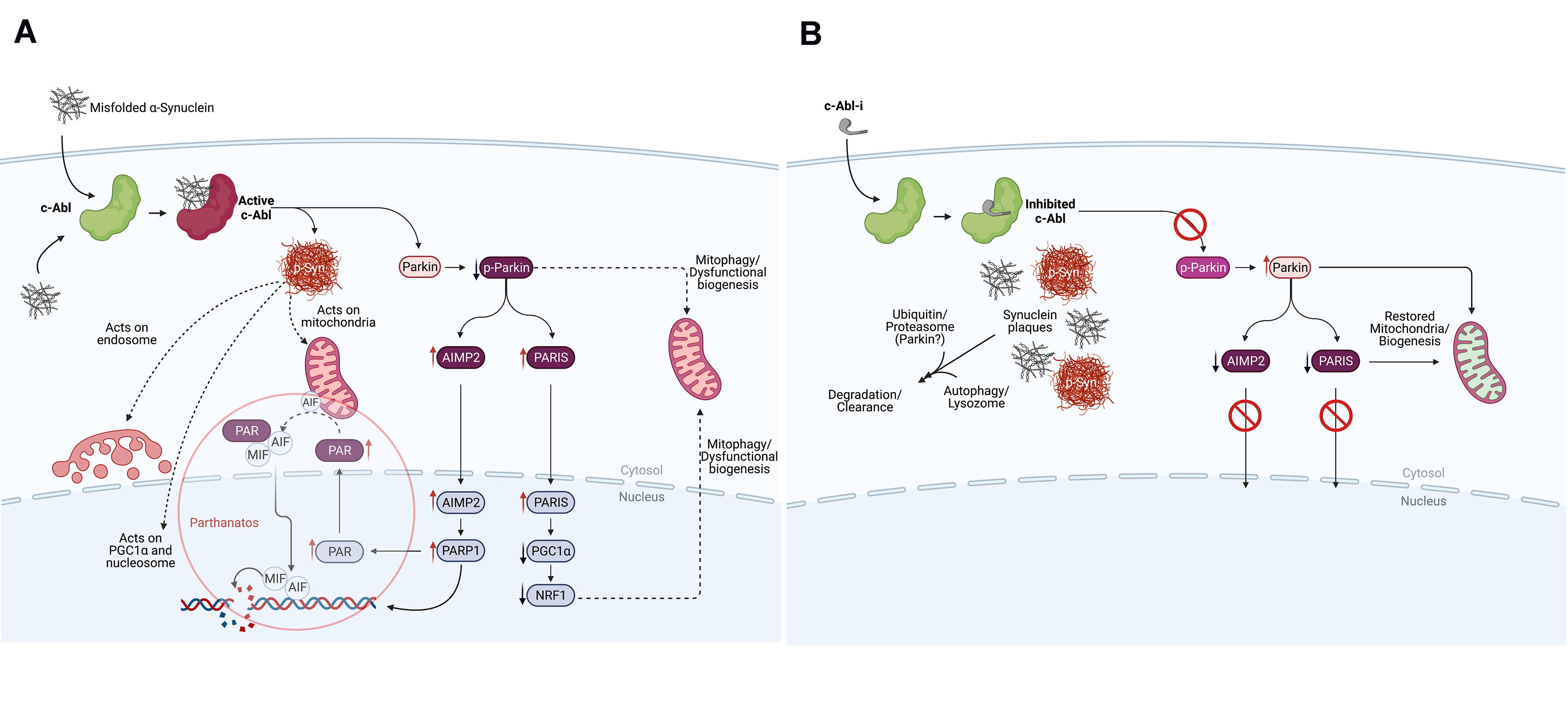
Background: PD pathology is characterized by the accumulation of misfolded alpha-synuclein. Animal models of PD demonstrate that internalization of misfolded alpha-synuclein activates c-Abl which phosphorylates alpha-synuclein and promotes alpha-synuclein pathology within the affected neurons. Additionally, c-Abl inactivates parkin, disrupting mitochondrial quality control and biogenesis, promoting neurodegeneration.
Method: Models of inherited or sporadic PD dependent on alpha-synuclein were used to evaluate therapeutic potential of the c-Abl inhibitor IkT-148009. These studies were followed by clinical assessment of the safety, tolerability and pharmacokinetics in older healthy human subjects and the assessment of the safety, tolerability, pharmacokinetics and therapeutic benefit of IkT-148009 in Parkinson’s patients.
Results: IkT-148009 once daily protected neurons, restored function, reduced pathological alpha-synuclein and suppressed markers of neuroinflammation in models. Daily oral administration in older and elderly healthy volunteers (N=88) between 12.5 mg and 325 mg for up to seven days was well-tolerated and exhibited linear dose proportionality, high systemic exposure, penetration into the CNS and only 3 adverse events which were not clinically significant. The safety and tolerability profile of IkT-148009 in healthy subjects was consistent with chronic toxicology studies in rat and monkey. PD patiewts with mild to moderate disease (Hoehn & Yahr < 3.0) displayed a similar profile of safety and tolerability. Measures of motor and non-motor features of disease displayed trends, but the trial is still blinded and ongoing as of this writing.
Conclusion: Orally administered IkT-148009 is a mechanistically-defined interventional therapy and a possible disease-modifying treatment. Clinically, IkT-148009 displayed a favorable safety and tolerability profile and was well-behaved pharmacologically. In patients, the ongoing Phase 1b trial is evaluating motor and non-motor features of disease, but with only a 7 day dosing period, no conclusions should be drawn from trends observed. These outcomes support further clinical evaluation of IkT-148009 for potential efficacy as a treatment or disease-modifying therapy.
References: (1) Werner MH and Olanow CW (2022) Parkinson’s Disease Modification through Abl Kinase Inhibition: An Opportunity. Mov. Disorders 37(1):6-15.
To cite this abstract in AMA style:
M. Werner, S. Karuppagounder, R. Rush, T. Kelly, T. Dawson, V. Dawson. IkT-148009 as a potential disease-modifying therapy in PD [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/ikt-148009-as-a-potential-disease-modifying-therapy-in-pd/. Accessed December 26, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/ikt-148009-as-a-potential-disease-modifying-therapy-in-pd/