Objective: To create a tissue-engineered construct with the dopaminergic (DA) cell numbers, axon lengths, and functionality required to reconstruct the human nigrostriatal pathway (NSP).
Background: Cell-based treatments for Parkinson’s disease (PD) have focused on transplants of DA neurons in the striatum, but this method disregards restoring the lost nigrostriatal fibers. In contrast, pathway reconstruction seeks to promote long-distance axon growth in vivo from implanted neurons in the nigra to reach the striatum. Repairing the circuitry with anatomical fidelity may reestablish proper regulation of striatal dopamine by restoring signaling between nigral neurons and adjacent/distant populations [1-3]. It is unclear if this growth could be achieved in humans. We have fabricated the tissue-engineered NSP (TE-NSP), which consists of an aggregate of DA neurons and its projected axon tracts encased in a protective hydrogel [4]. TE-NSPs are created/optimized in vitro to mimic the NSP before being implanted as a unit to rebuild it. We sought to build TE-NSPs based on clinical criteria, particularly the ~100,000 neurons and ~2 cm-long axons needed to repair the human NSP [5].
Method: The hydrogel was made by photocrosslinking methacrylated hyaluronic acid within a tube containing a needle, forming a micro-column with inner/outer diameters of 500/973 µm. A collagen/laminin solution was gelled within the lumen to enable axon growth. Aggregates of human iPSC-derived DA neurons were seeded within one end of the lumen, and they extended axons through the matrix over time.
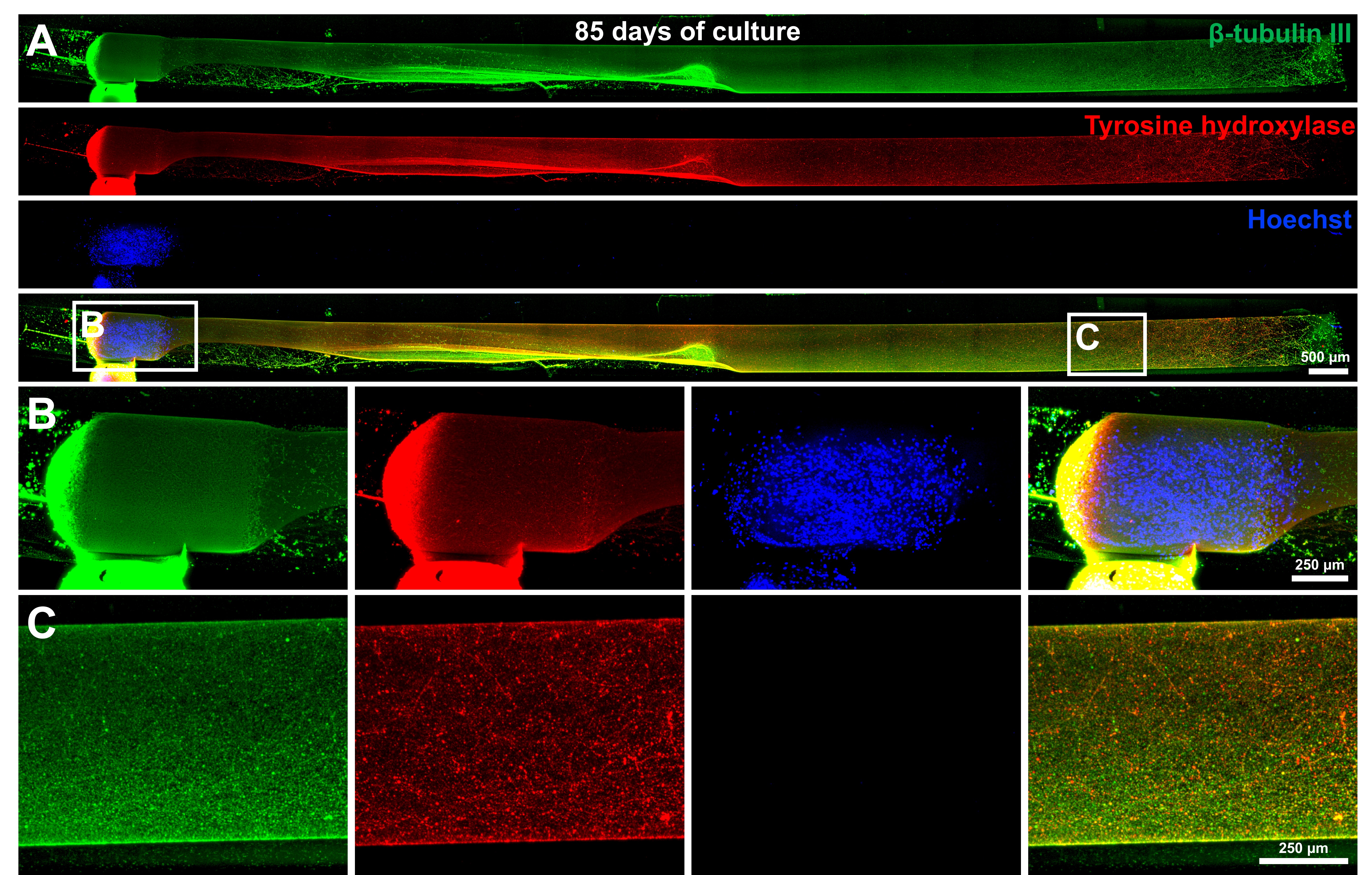
Results: TE-NSPs were fabricated with an aggregate containing 100,000 human DA neurons, reaching maximum growth rates of 0.5-0.8 mm/day. The aggregate projected long, dense, tyrosine hydroxylase+ axons that populated the lumen of the micro-column. Fast-scan cyclic voltammetry indicated both somatodendritic and axonal dopamine release in TE-NSPs (270-400 nM after electrical stimulation). The axons could integrate with a striatal aggregate and release dopamine within. We obtained full axon growth in 1.7 cm-long micro-columns [figure1], and we anticipate greater lengths in longer columns.
Conclusion: TE-NSPs have the potential to be scaled for human implantation, while exhibiting structural/functional features of the NSP. We are currently optimizing the biofabrication process and envisioning preclinical studies in models of PD.
References: [1] C. R. Lee, J. M. Tepper, Basal ganglia control of substantia nigra dopaminergic neurons. J. Neural Transm. Suppl., 71–90 (2009). [2] M. E. Rice, J. C. Patel, Somatodendritic dopamine release: recent mechanistic insights. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370 (2015). [3] A. Faynveitz, H. Lavian, A. Jacob, A. Korngreen, Proliferation of Inhibitory Input to the Substantia Nigra in Experimental Parkinsonism. Front. Cell. Neurosci. 13, 417 (2019). [4] L. A. Struzyna, K. D. Browne, Z. D. Brodnik, J. C. Burrell, J. P. Harris, H. Isaac Chen, J. A. Wolf, K. V. Panzer, J. Lim, J. E. Duda, R. A. España, D. Kacy Cullen, Tissue engineered nigrostriatal pathway for treatment of Parkinson’s disease. J. Tissue Eng. Regen. Med. 12 (2018). [5] P. Hagell, P. Brundin, Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J. Neuropathol. Exp. Neurol. 60, 741–752 (2001).
To cite this abstract in AMA style:
W. Gordián Vélez, D. Chouhan, H I. Chen, J. Duda, D K. Cullen. Tissue-engineered nigrostriatal pathway to rebuild dopaminergic axons in Parkinson’s disease based on clinical design criteria [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/tissue-engineered-nigrostriatal-pathway-to-rebuild-dopaminergic-axons-in-parkinsons-disease-based-on-clinical-design-criteria/. Accessed December 16, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/tissue-engineered-nigrostriatal-pathway-to-rebuild-dopaminergic-axons-in-parkinsons-disease-based-on-clinical-design-criteria/