Category: Technology
Objective: Summarize the findings from 3 developmental studies of the Parkinson’s Disease Functional Impacts Digital Instrument (PD-FIDI) – a novel endpoint for assessing PD symptoms.
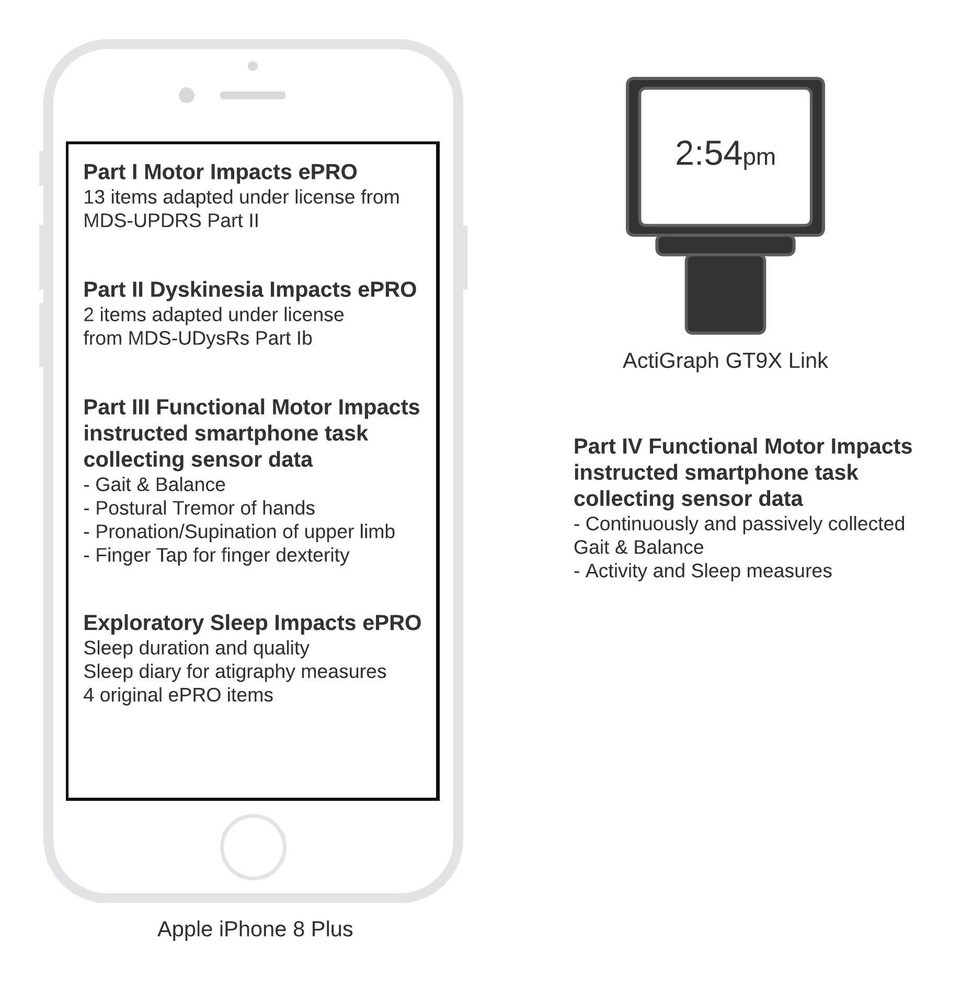
Background: PD-FIDI was developed to objectively assess PD symptoms in patients at home. PD-FIDI includes an iPhone application with electronic patient-reported outcomes (ePROs) and functional assessments, plus a wrist-worn device (ActiGraph GT9X) [figure1]. We report results of three studies that, in line with FDA guidance, assess PD-FIDI’s analytical validity, safety, and usability prior to clinical validation.
Method: Study One had 2 objectives: To determine a) accelerometer accuracy for iPhone and GT9X compared with a Quanser Shake Table II [1] for accelerations observed in walking and tremor; and b) analytical validity and operational tolerance (deviation from instruction) of functional assessments and wrist-worn measures in 11 healthy volunteers compared with the observations of 3 trained raters. Agreement was measured via intraclass correlation coefficient (ICC) for assessments, and mean absolute percentage error (MAPE) was calculated for wrist-worn measures. Study Two covered expert review and interviews with 5 PD patients to assess comprehension and usability of PD-FIDI’s 2 ePROs, adapted from MDS–Unified PD Rating Scale Part II and MDS–Unified Dyskinesia Rating Scale Part Ib. Study Three assessed PD-FIDI’s safety and usability in a 2-phase formative human factors study with 9 PD patients.
Results: For Study One, 99% of the ICC for iPhone and 92% of ICC for GT9X were acceptable (ICC ≥0.75) for acceleration >0.1 g. After optimization, ICC for 17 of 21 functional assessment measures were acceptable (ICC ≥0.75). For wrist-worn measures, MAPE was <6%. In Study Two, expert and patient reviews resulted in a number of minor changes to improve usability and comprehension of PD-FIDI’s digital PROs. In Study Three – a two-phase formative human factors study – phase 1 revealed the need for minor user experience improvements, which were included and tested in phase 2 to confirm PD-FIDI’s usability and safety.
Conclusion: The results of the 3 studies suggest that the PD-FIDI device and application show sufficient sensor precision, analytical validity (ICC ≥0.75), and usability to support use in a clinical validation study (OBS16136 – now enrolling patients).
References: [1] https://www.quanser.com/products/shake-table-ii/
To cite this abstract in AMA style:
A. Pearlmutter, N. Wong, M. Levin, R. Zheng, R. Ellis, P. Kelly, M. Peterschmitt. Parkinson’s Disease Functional Impacts Digital Instrument (PD-FIDI): Studies Preceding Clinical Validation [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/parkinsons-disease-functional-impacts-digital-instrument-pd-fidi-studies-preceding-clinical-validation/. Accessed February 5, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/parkinsons-disease-functional-impacts-digital-instrument-pd-fidi-studies-preceding-clinical-validation/