Category: Surgical Therapy: Parkinson's Disease
Objective: To evaluate the location of therapeutic electrode contacts and correlate those with the clinical outcomes of deep brain stimulation (DBS) of the globus pallidus (GP) to treat Parkinson’s disease (PD).
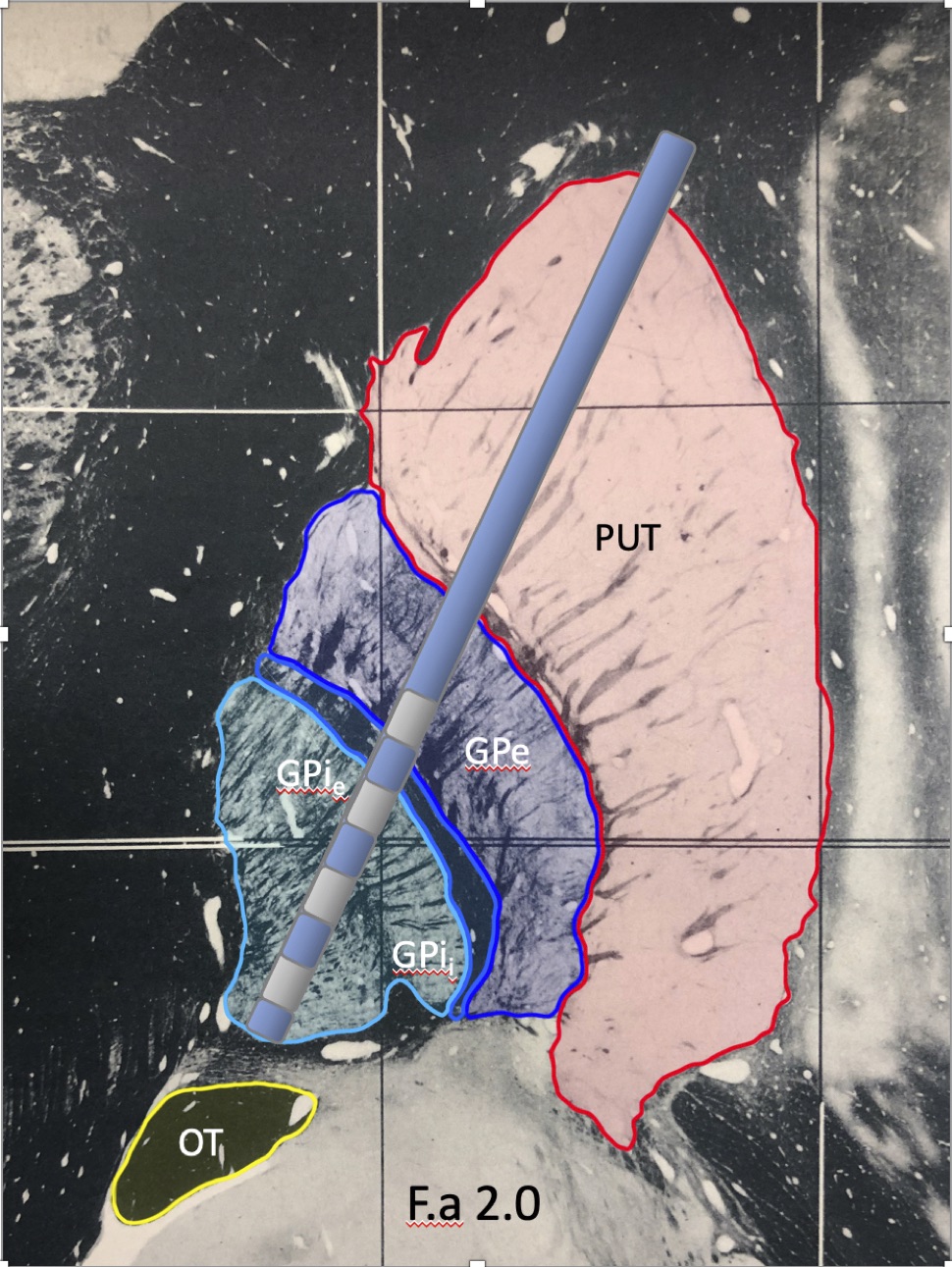
Background: The internal globus pallidus (GPi) has been a traditional surgical target for patients with medication-refractory PD for management of bradykinesia, rigidity, tremor, and dyskinesia. Optimization of the clinical benefit of DBS within the globus pallidus is currently an interactive process of refining stimulation over the first six months of DBS therapy.
Method: A retrospective review identified 56 patients who underwent bilateral GP-DBS implantation at our institution from January 2015 to January 2020. MRI sequences were obtained pre-surgically for planning. Post-implantation CT was obtained intra-operatively and merged with pre-surgical MRI scans. Patients were followed at our institution for stimulation programming and completed pre- and post-surgery clinical outcome assessments. We examined the correlation between the therapeutic electrodes’ location following the six-month programming session and pre- and post-surgery clinical outcomes (UPDRS part III, dyskinesia score, and levodopa equivalent daily dose (LEDD)).
Results: Out of the 112 leads implanted in 56 patients, the therapeutic cathode was most frequently located in the lamina between the external segment of GPi (GPie) and GPe (n=40). Other common cathode locations included the GPe (n=24), GPie (n=15), and the lamina between GPIi/GPie (n=14). GPIi was a less frequent location (n=9), followed by the lamina between GPe and putamen (n=5), putamen (n=2), and subpallidum (n=2). The majority of patients (73%) were programmed with a monopolar configuration. Post-surgery UPDRS III OFF medications, ON stimulation scores (z=-4.02, p<0.001) significantly improved, as did post-surgery dyskinesia ON scores (z=-4.08, p<0.001) and post-surgery LEDD (z=-4.7, p<0.001).
Conclusion: Though most DBS centers consider ventral GP (GPi) an important target for DBS, few prior studies have examined whether the dorsal GP (GPe) may be a more effective target for treating motor symptoms of PD1,2. Our assessment of therapeutic contact locations and associated clinical outcomes supports the conclusion that the lamina between GPi and GPe, or GPe itself, may be the optimal target.
References: 1. Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp Neurol. 2012 Jan;233(1):581-6. doi: 10.1016/j.expneurol.2011.09.031. Epub 2011 Oct 1. PMID: 22001773; PMCID: PMC3536483. 2. Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay RA. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Mov Disord. 2004;19:907–915.
To cite this abstract in AMA style:
J. Jiao, M. Holland, A. Mitchell, D. Safarpour, K. Burchiel. Retrospective Review of Globus Pallidus Deep Brain Stimulation Electrode Contact Locations and Clinical Outcomes in Parkinson’s Disease [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/retrospective-review-of-globus-pallidus-deep-brain-stimulation-electrode-contact-locations-and-clinical-outcomes-in-parkinsons-disease/. Accessed April 2, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/retrospective-review-of-globus-pallidus-deep-brain-stimulation-electrode-contact-locations-and-clinical-outcomes-in-parkinsons-disease/