Category: Rating Scales
Objective: To implement the first 3 phases of the scale development process that will create the PD-MAS.
Background: Drug therapy is a central strategy in the control of Parkinson’s disease (PD) symptoms. Measuring behaviors related to medication adherence, specifically in patients with PD, is vital to understanding therapeutic response and effectiveness, and to providing valid and reliable clinical and research results.
Method: This is an ongoing, clinimetric, multi-methods and multicenter international study. Of the total of 6 phases, 3 were developed: Phase 1) Formulation of the purpose statement of the scale and target group; Phase 2) Definition of the subdomains of interest of the scale conducted through the triangulation of data observed from 3 sources: scientific literature, medication adherence scales used in PD patients and focus groups of PD patients and care partners; Phase 3) Definition of the specific objectives of the scale.
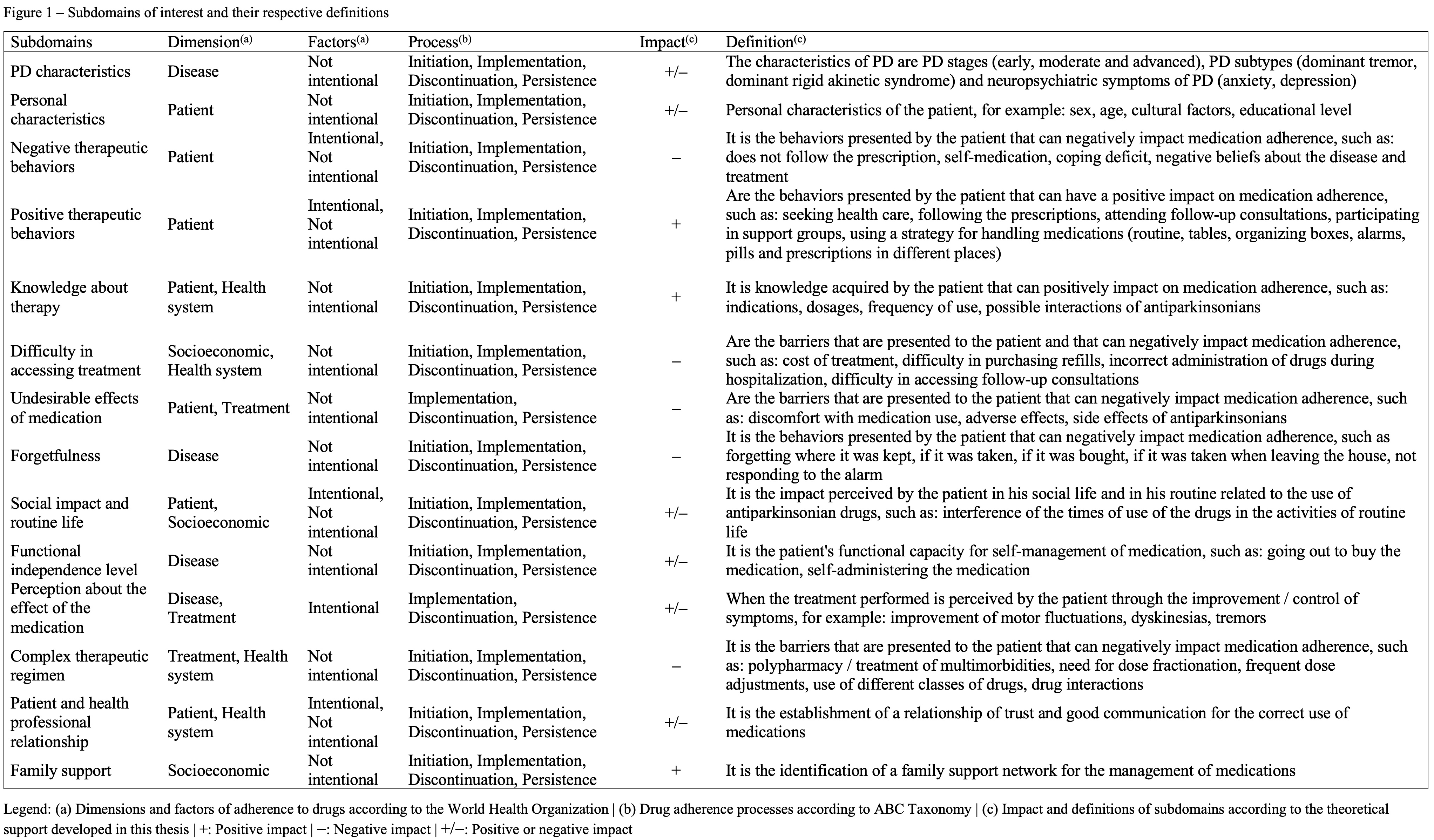
Results: Phase 1: PD-MAS is being developed for patients with PD, directly or indirectly involved in the management of antiparkinsonian medications to determine the level of adherence to antiparkinsonian medications by measuring intentional and nonintentional factors related to the dimensions (patient, treatment, health and socioeconomic systems) involved in the medication adherence process (initiation, implementation, discontinuation and persistence). In cases where patients are indirectly involved or are totally dependent on others for the management of medications, the care partner will be the target group. Phase 2: We identified 14 subdomains of interest, encoded through 322 phenomena identified in the 3 sources (literature n=30, Scales n=60, Patients n=117, and care partners n=115), 50% with “Very strong” triangulation level, and none considered as “Weak”. The 14 subdomains were classified and defined as described in figure 1. Phase 3: Based on the data triangulation, 5 PD-MAS objectives were established according to the impact that multidimensional and multifactorial characteristics have on each subdomain.
Conclusion: The completion of the first 3 phases of the scale development process made it possible to determine its purpose, the target group, the subdomains of interest and the specific objectives, considering phenomena originating not only from scientific literature but also from patients and their care partners. These phases will support the creation of the PD-MAS items and response options.
To cite this abstract in AMA style:
M. Tosin, C. Goetz, G. Stebbins, B. Oliveira. Development of the Parkinson’s Disease Medication Adherence Scale (PD-MAS) [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/development-of-the-parkinsons-disease-medication-adherence-scale-pd-mas/. Accessed April 4, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/development-of-the-parkinsons-disease-medication-adherence-scale-pd-mas/