Category: Rare Genetic and Metabolic Diseases
Objective: To evaluate neurological efficacy outcomes of ALXN1840 treatment in adults with Wilson disease (WD) from a phase 2, open-label, single-arm study (NCT02273596).
Background: WD is a rare disorder of copper (Cu) metabolism resulting in hepatic, neurological and psychiatric manifestations. Current therapies may not always improve neurological symptoms and may cause paradoxical worsening. ALXN1840 is a novel Cu- and protein-binding agent in development for the treatment of WD.
Method: Eligible participants had WD, non-ceruloplasmin-bound Cu concentration ≥0.8 µmol/L and were aged ≥18 years. Patients who had received prior WD treatment for 29 days–24 months were allocated to Cohort 1, and those who were treatment-naive or had received prior WD treatment for ≤28 days to Cohort 2. Patients received ALXN1840 monotherapy with response-guided individualized dosing (15–60 mg/day) for ≤42 months. We analyzed changes from Baseline in Unified Wilson Disease Rating Scale (UWDRS) parts II (patient-reported disability) and III (investigator-reported neurological status) scores, and investigator-reported Mini International Neuropsychiatric Interview (M.I.N.I.) scores. Adverse events (AEs) were recorded.
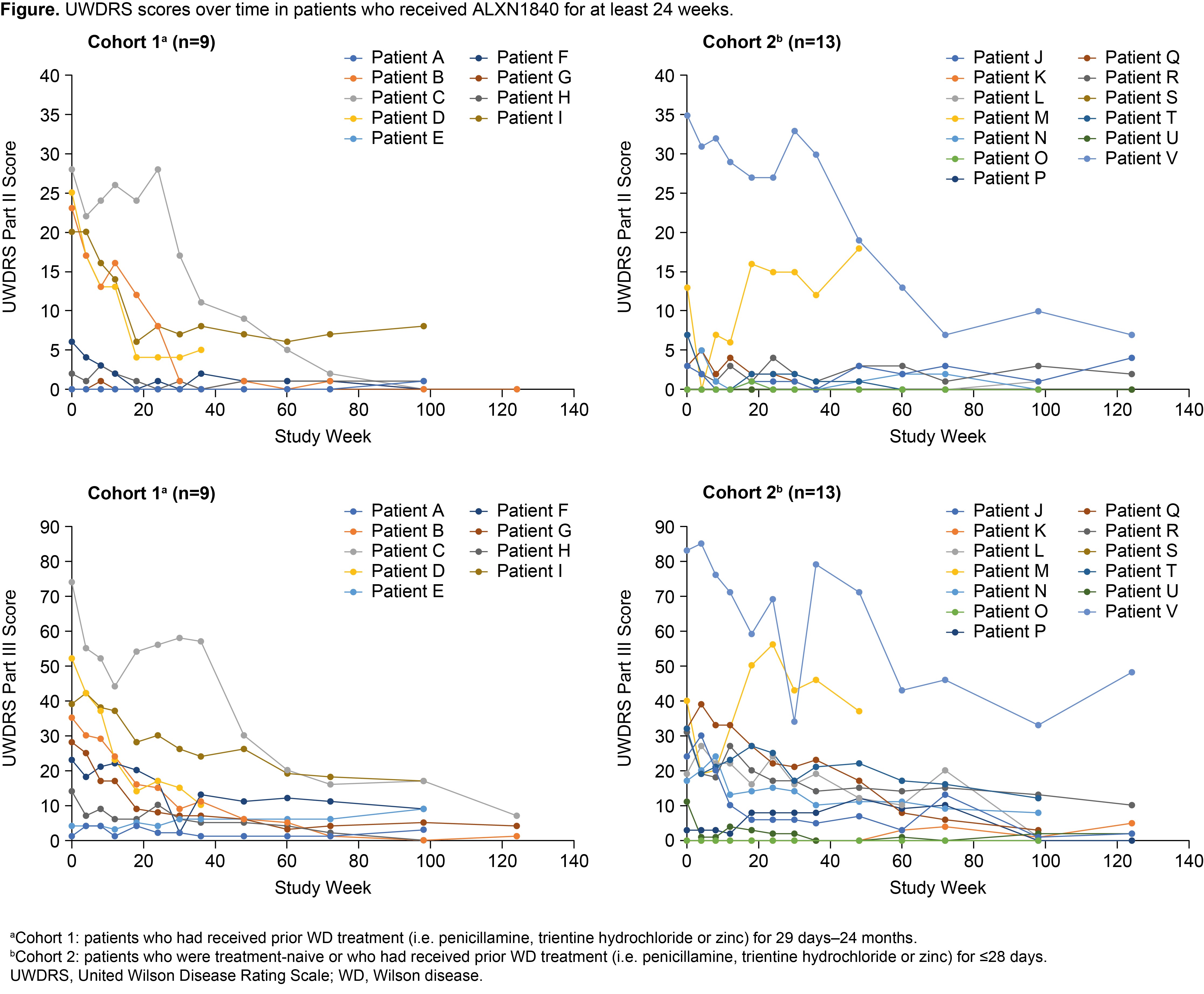
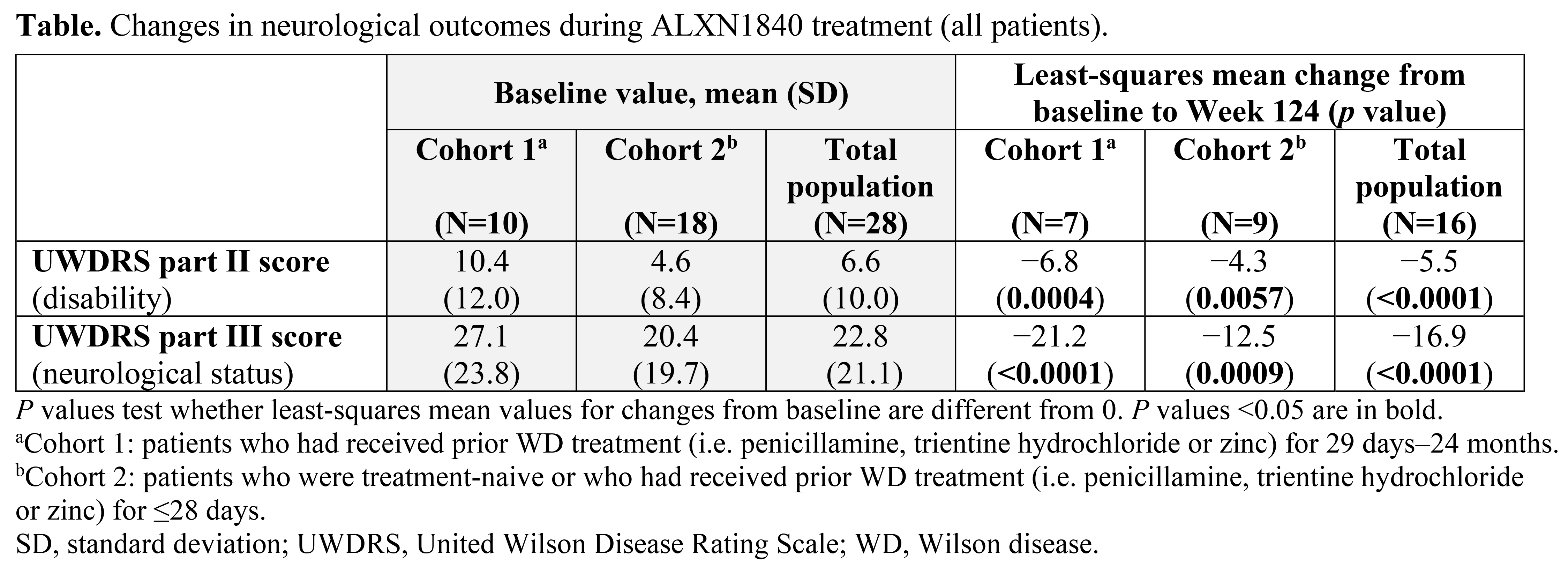
Results: Twenty-eight patients received ALXN1840 (10 in Cohort 1 and 18 in Cohort 2). At Baseline, 16/28 (57%) and 25/28 patients (89%) had abnormal UWDRS parts II and III scores (>0), respectively. Significant improvements (p<0.05) from Baseline to Week 124 were seen in UWDRS parts II and III scores for the total population (N=16) and both cohorts [Table, Figure]. Most patients had psychiatric manifestations at Baseline (25/28 [89%] had M.I.N.I. scores >0); some M.I.N.I. items improved during ALXN1840 treatment.
Three patients in Cohort 2 experienced neurological serious AEs. One initially presented with marked personality disorder (Week 9), followed by gait disturbance (Week 14) and neuropsychiatric deterioration (Week 15), before discontinuing (Week 29). The other two had milder deteriorations at Weeks 16 and 46 and discontinued at Weeks 20 and 56, respectively. Three patients discontinued owing to non-neurological AEs (hepatic enzyme elevation [n=3] and agranulocytosis [n=1]).
Conclusion: ALXN1840 led to significant improvements from Baseline in neurological outcomes, supporting its efficacy in patients with WD.
To cite this abstract in AMA style:
A. Członkowska, S. Moseley, E. Swenson, D. Bega, P. Hedera, D. Nicholl, J. Bronstein. Impact of ALXN1840 on the neurological symptoms of Wilson disease: secondary outcomes of a phase 2, open-label, single-arm study [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/impact-of-alxn1840-on-the-neurological-symptoms-of-wilson-disease-secondary-outcomes-of-a-phase-2-open-label-single-arm-study/. Accessed April 4, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-of-alxn1840-on-the-neurological-symptoms-of-wilson-disease-secondary-outcomes-of-a-phase-2-open-label-single-arm-study/