Category: Parkinson's Disease: Pathophysiology
Objective: The objective of this study is to analyze the effect of exposure to AT1 receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) on the clinical progression of parkinson’s disease (PD) of newly diagnosed PD patients from the Parkinson’s Progress Marker Initiative (PPMI) study database.
Background: Parkinson’s disease (PD) is the second leading neurodegenerative disorder after Alzheimer’s [1]. The many advances in the pathophysiology of PD have not yielded either preventing or progression-retarding treatments. The renin-angiotensin system (RAS) in the brain was reported in regulating dopaminergic neurotransmission and neuron survival. Angiotensin II AT1 receptor blockers (ARBs) and the angiotensin-converting enzyme inhibitors (ACEIs) prevented neuronal damage caused by dopaminergic neurotoxins in experimental PD cellular and animal models [2]. Furthermore, perindopril, an ACEI, reduced the latency of the motor response to L-DOPA and increased “on” periods during the waking day in a small double-blind, randomized, cross-over study [3]. We analyzed data of the Parkinson’s Progression Markers Initiative (PPMI) study and evaluated the potential effects of ARBs and ACEIs on disease progression in PD patients.
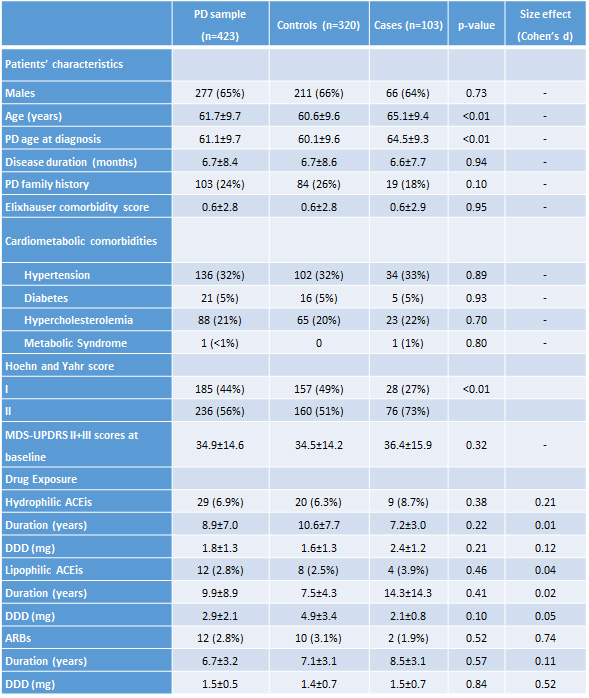
Method: We included 423 newly diagnosed PD patients, free from antiparkinsonian treatment, from the PPMI. We compared the proportion of patients starting on L-DOPA during the first year of follow-up, and the changes in MDS-UPDRS total score and sub-scores during the first five follow-up years for patients exposed or not to ARBs or ACEIs.
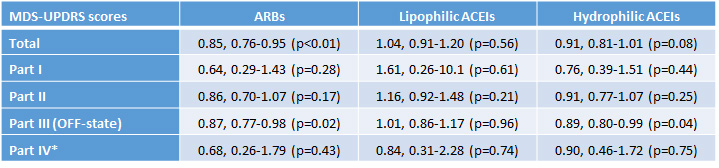
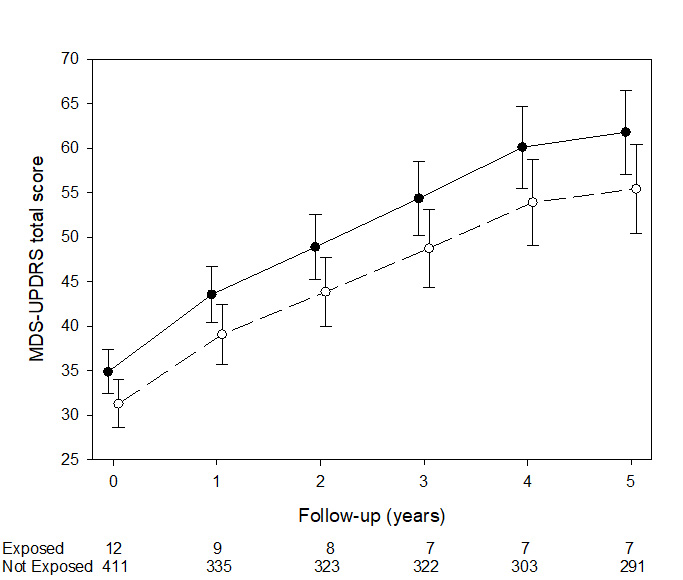
Results: Treatment with ARBs did not affect the proportion of patients on L-DOPA during the first year, (adjusted OR, 95% CI= 0.26, 0.03-2.18, p=0,52), while reduced MDS-UPDRS total score during the follow-up in patients (0.85, 0.76-0.95, p<0.01). Patients treated with ACEIs experienced no changes in either measure.
Conclusion: We observed a signal of potential clinical effects of the ARBs that deserves further attention in future clinical trials. The ARBs are commonly used in PD patients for treating hypertension. Then, Phase I studies may not be needed, and a proof-of-concept futility trial seems warranted. The outcome of our study may lay the bases for the corresponding design.
References: [1] E.R. Dorsey et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030, Neurology 68(5) (2007) 384-6. [2] S. Perez-Lloret et al. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease, Expert opinion on investigational drugs 26(10) (2017) 1163-1173. [3] K.A. Reardon et al. The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson’s disease, Australian and New Zealand journal of medicine 30(1) (2000) 48-53.
To cite this abstract in AMA style:
L. Udovin, M. Otero-Losada, S. Bordet, G. Chevalier, C. Quarracino, F. Capani, S. Perez-Lloret. Effects of Angiotensin Type 1 Receptor Antagonists on Parkinson’s Disease Progression: an exploratory study in the PPMI database [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/effects-of-angiotensin-type-1-receptor-antagonists-on-parkinsons-disease-progression-an-exploratory-study-in-the-ppmi-database/. Accessed January 7, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/effects-of-angiotensin-type-1-receptor-antagonists-on-parkinsons-disease-progression-an-exploratory-study-in-the-ppmi-database/