Category: Parkinson's Disease: Neurophysiology
Objective: To test the hypothesis that pallidocortical local field potential (LFP) beta oscillations encode movement vigor.
Background: Recent work suggests the motor basal ganglia adjusts “movement vigor” (the speed and amplitude of movements) based on motivational context [1-3]. The hypokinetic state in Parkinson disease (PD) may result from a loss of movement vigor due to the integral role of striatal dopamine in controlling vigor [4-7]. PD motor symptom severity is associated with exaggerated beta oscillations in the basal ganglia thalamocortical network. We therefore hypothesized that pallidocortical beta oscillations would scale with movement vigor.
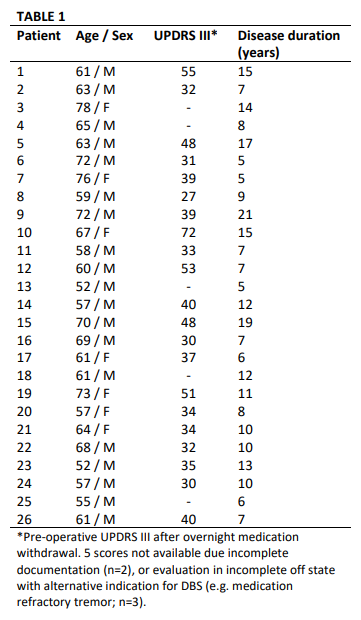
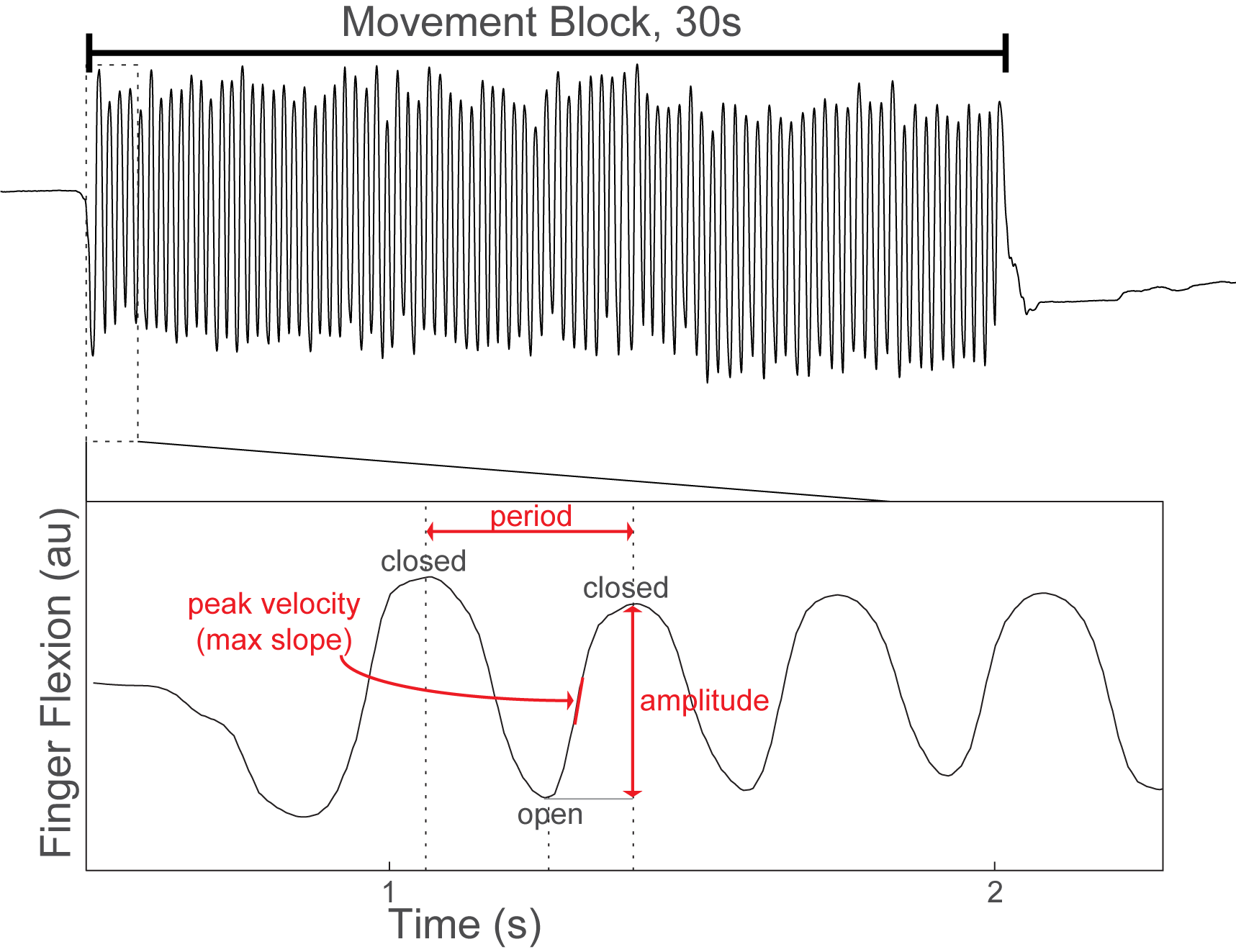
Method: 26 patients undergoing GPi DBS surgery for treatment of PD performed alternating 30-second blocks of rest and repetitive hand open/closing movements with simultaneous recording of contralateral GPi LFPs and motor cortex (M1) electrocorticography. [table1] Finger flexion was recorded with a kinematic data glove. Glove data was used to segment LFPs into movement cycles and to calculate movement amplitude, velocity and cycle period. [figure1] Using Morlet wavelet time frequency analysis we examined local spectral power and GPi-M1 phase locking value (PLV) during movement cycles and their within-subject correlation with movement kinematics. Power and PLV were also compared across movement blocks with hastening (decreasing cycle duration over time) and decrement (decreasing amplitude over time).
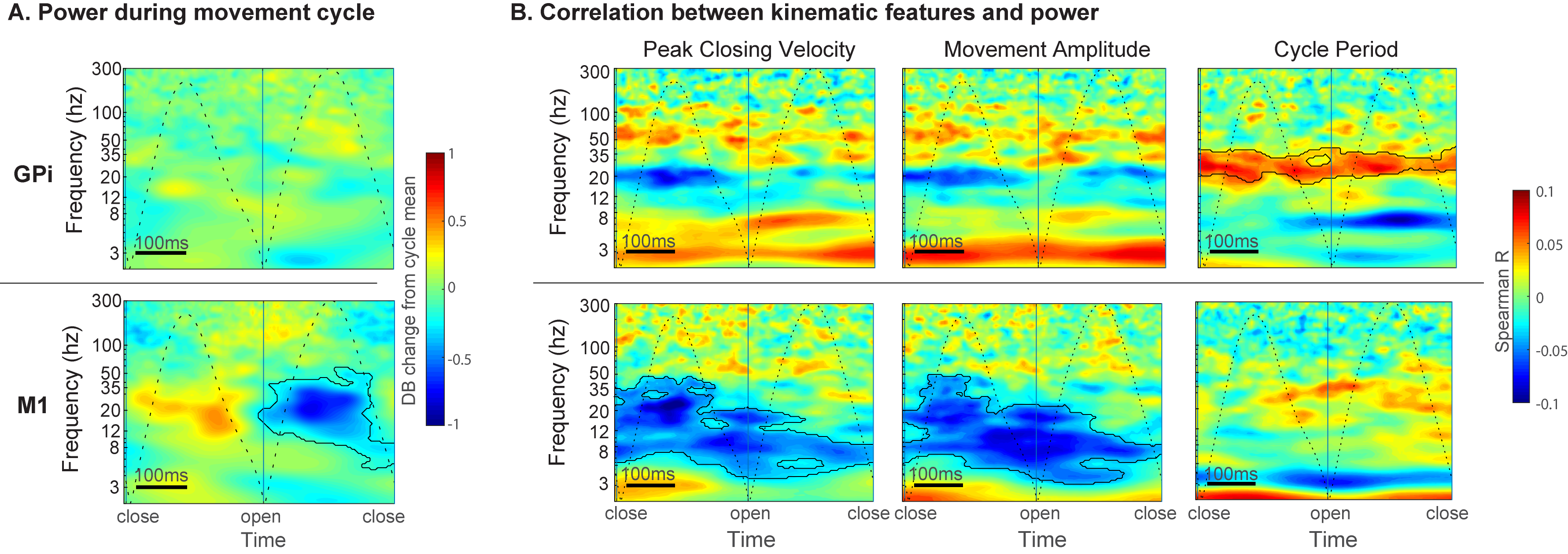
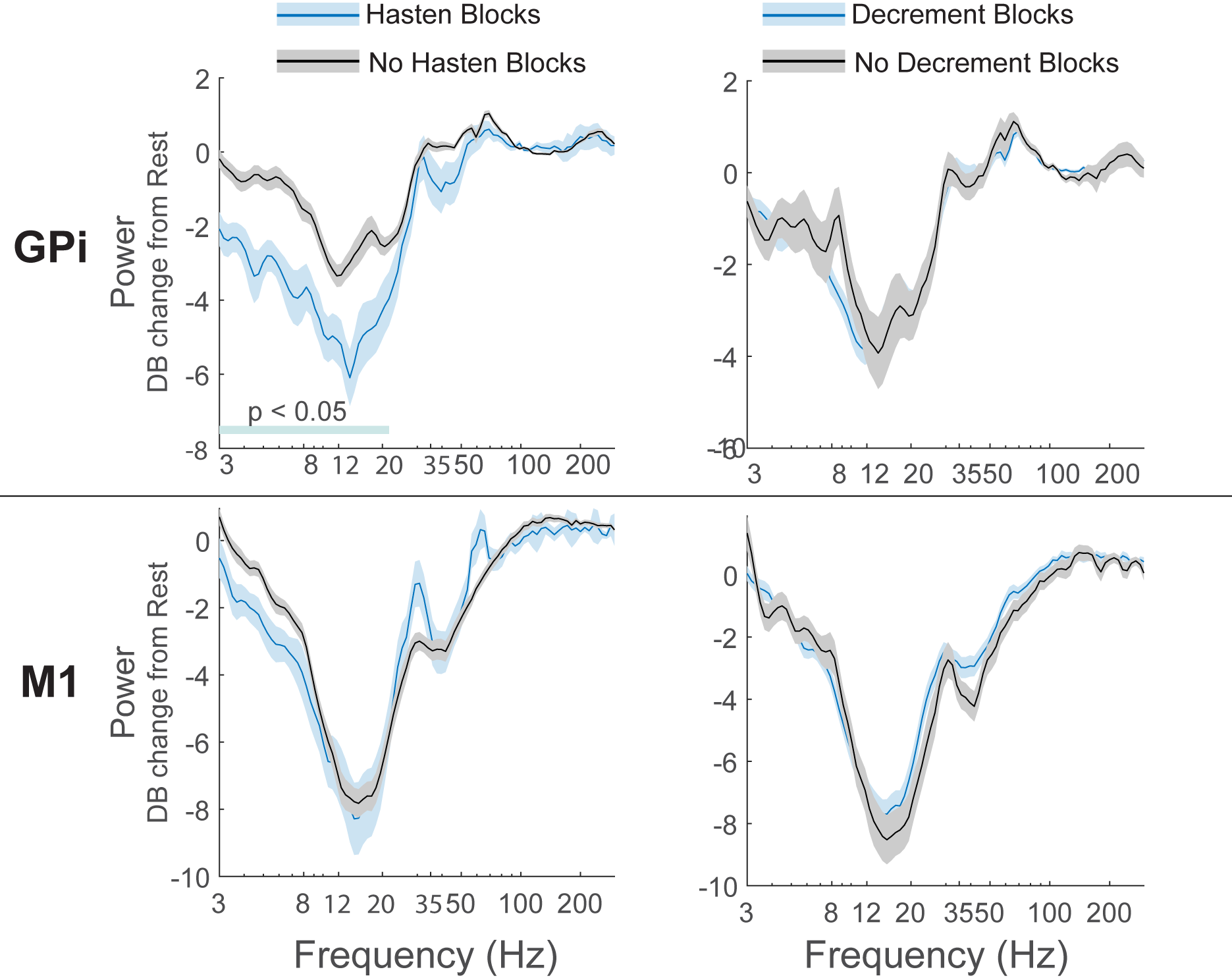
Results: M1 alpha and beta power is modulated by phase of movement, with relative suppression during the closing phase. Peak amplitude and velocity were negatively correlated with alpha and low beta (8-20 Hz) power across cycles. In contrast, GPi power was not significantly modulated by phase of movement, but high beta (20-35 Hz) power was positively correlated with cycle duration. [figure2] Progressive decrease in cycle duration with repeated movement (hastening) was associated with reduced beta power in the GPi. [figure3] There were no significant relationships between GPi-M1 PLV and movement kinematics.
Conclusion: Movement kinematics and vigor are differentially encoded by GPi and M1. Greater amplitude and velocity of ongoing movements was associated with reduced beta power in motor cortex. In contrast, during repetitive movements GPi beta power encodes movement gain across a longer timescale with higher power being associated with slower movement cycles.
References: [1] E. A. Yttri and J. T. Dudman, “Opponent and bidirectional control of movement velocity in the basal ganglia,” Nature, vol. 533, pp. 402–406, May 2016. [2] R. S. Turner and M. Desmurget, “Basal ganglia contributions to motor control: a vigorous tutor,” Curr. Opin. Neurobiol., vol. 20, no. 6, pp. 704–716, Dec. 2010. [3] J. T. Dudman, J. W. Krakauer, A. Karpova, and R. Kiani, “The basal ganglia: from motor commands to the control of vigor This review comes from a themed issue on Neurobiology of cognitive behavior,” Curr. Opin. Neurobiol., vol. 37, pp. 158–166, 2016. [4] B. Panigrahi et al., “Dopamine Is Required for the Neural Representation and Control of Movement Vigor,” Cell, vol. 162, no. 6, pp. 1418–1430, Sep. 2015. [5] E. A. Yttri and J. T. Dudman, “A Proposed Circuit Computation in Basal Ganglia: History-Dependent Gain,” Mov. Disord., vol. 33, no. 5, pp. 704–716, May 2018. [6] P. Mazzoni, A. Hristova, and J. W. Krakauer, “Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation.,” J. Neurosci., vol. 27, no. 27, pp. 7105–16, Jul. 2007. [7] M. A. Carland, D. Thura, and P. Cisek, “The Urge to Decide and Act: Implications for Brain Function and Dysfunction:,” https://doi.org/10.1177/1073858419841553, p. 107385841984155, 2019.
To cite this abstract in AMA style:
K. Cross, E. Koenig, J. Choi, N. Pouratian. Movement vigor is differentially encoded in motor cortex and globus pallidus in Parkinson disease [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/movement-vigor-is-differentially-encoded-in-motor-cortex-and-globus-pallidus-in-parkinson-disease/. Accessed April 22, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/movement-vigor-is-differentially-encoded-in-motor-cortex-and-globus-pallidus-in-parkinson-disease/