Objective: Analyze the impact of levodopa-carbidopa intestinal gel (LCIG) treatment on dyskinesia duration and severity in advanced Parkinson’s disease (APD) patients participating in the COSMOS study with either <4 hours or ≥4 hours of dyskinesia at baseline.
Background: Approximately 40% of APD patients present with troublesome dyskinesia symptoms after 4 years of oral levodopa treatment [1]. Continuous delivery of LCIG has been shown to improve motor complications including dyskinesia.
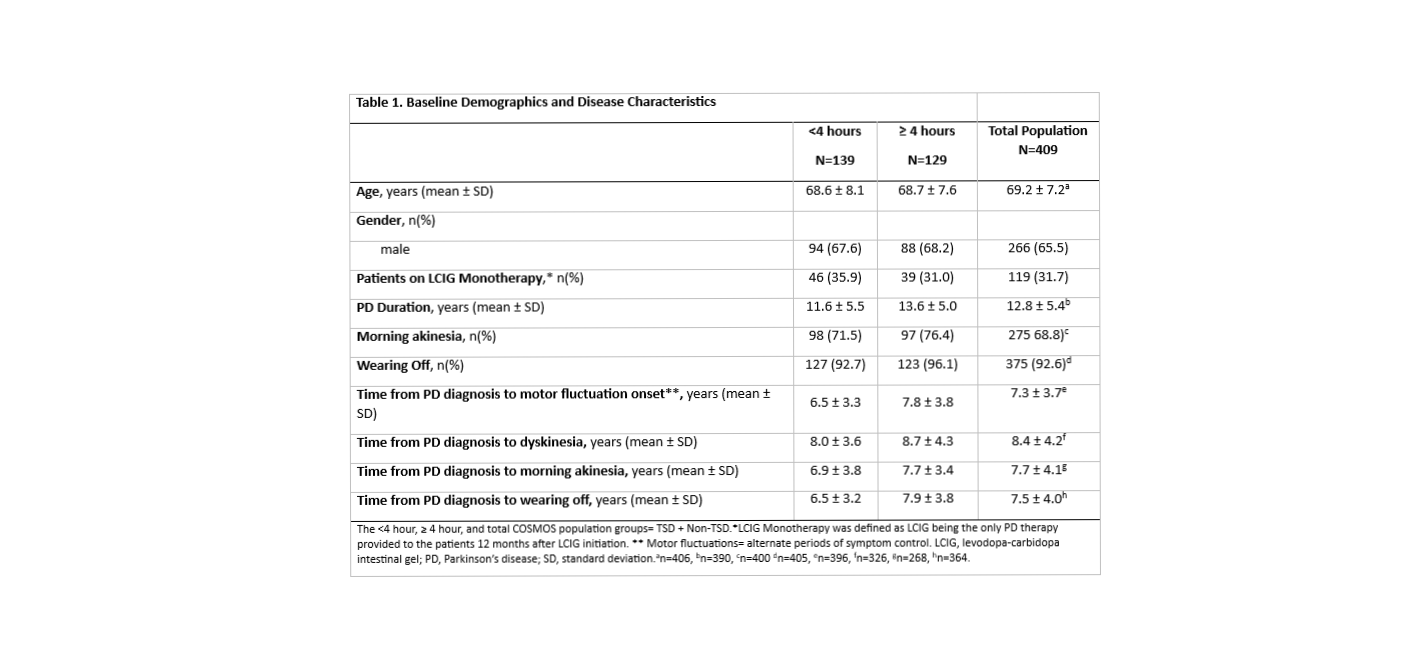
Method: COSMOS (COmedication Study assessing Mono- and cOmbination therapy with levodopa-carbidopa inteStinal gel) is a multi-country, cross-sectional, retrospective and observational study of APD patients treated with LCIG for ≥ 12 months (NCT03362879). This post-hoc analysis assessed patient demographics, disease characteristics, and dyskinesia severity (troublesome [TSD] and non-TSD), duration and pain in patients with <4 hours and ≥4 hours of dyskinesia before LCIG initiation. Dyskinesia duration, severity and pain were correlated with patient reported outcomes (PROs). The PROs were quality of life (Parkinson’s Disease Questionnaire-8[PDQ‑8]), non-motor symptoms (NMSS Total Score), and sleep (Parkinson’s disease sleep scale-2 [PDSS-2]).
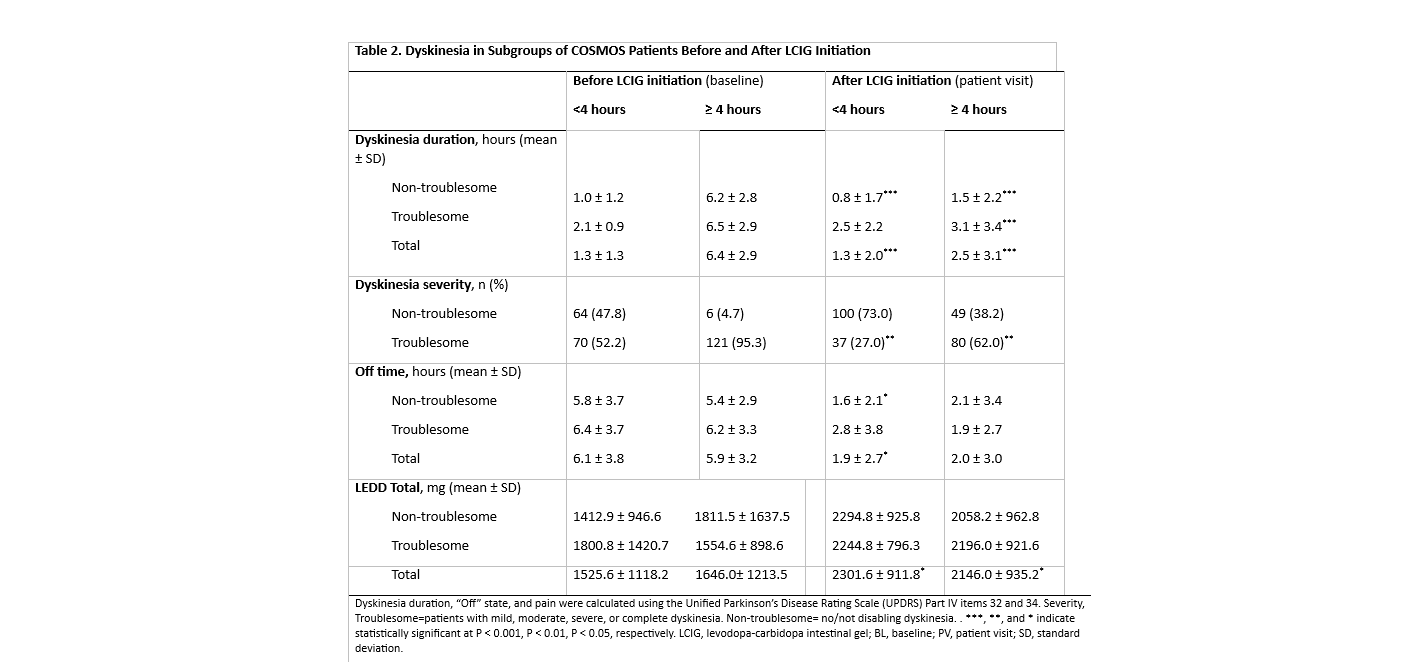
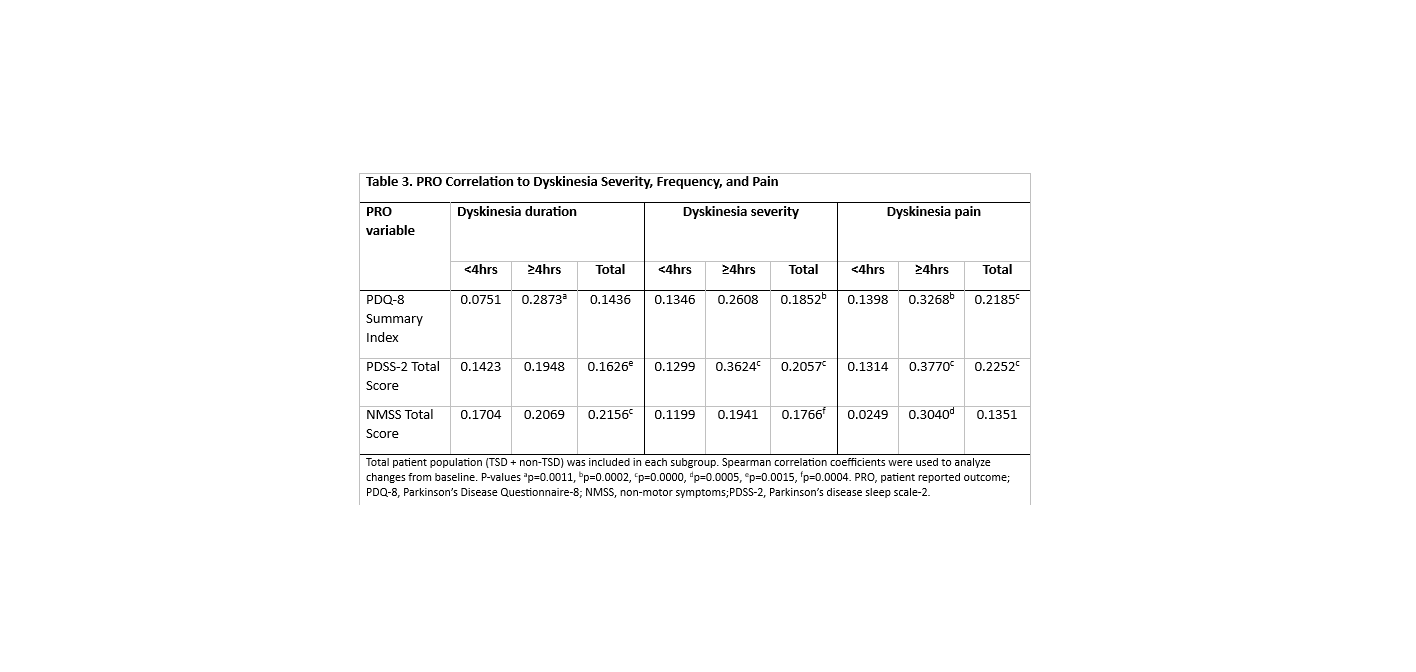
Results: Of 268 patients, 139 (51.9%) had <4 hours of dyskinesia and 129 (48.1%) had ≥ 4 hours before LCIG initiation. Patient demographics and disease characteristics were similar between subgroups at patient visit (Table 1). In the ≥4 hours subgroup, both the TSD and non-TSD groups had significantly decreased duration (p< 0.001) from baseline (BL) to patient visit. In both subgroups, the percentage of patients who suffered from TSD dyskinesia also significantly decreased (p<0.01) from BL to patient visit (Table 2). Although weak, there were significant positive correlations between dyskinesia duration, severity and pain and PDQ‑8, NMSS, and PDSS-2 for the ≥4hours subgroup (Table 3). Safety data were consistent with the known LCIG profile.
Conclusion: Dyskinesia severity and duration improved after LCIG treatment, despite the duration at BL. The observed improvements in dyskinesia also have a positive correlation with QoL, NMSS, and sleep, suggest that LCIG treatment may benefit patients with ≥4 hours of dyskinesia at BL. The correlation was stronger as dyskinesia duration, severity and pain worsened.
References: [1] Antonini, A., et al. Oral and Infusion Levodopa-Based Strategies for Managing Motor Complications in Patients with Parkinson’s Disease. CNS Drugs 24, 119–129 (2010).
To cite this abstract in AMA style:
A. Fasano, C. Spanaki, T. Gurevich, M. Simu, L. Bergmann, J. Parra, P. Sanchez Vega, O. Sanchez-Soliño, N. Kovács. Impact on Dyskinesia in Advanced Parkinson’s Disease Patients Treated with LCIG: Analysis from the COSMOS Study [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/impact-on-dyskinesia-in-advanced-parkinsons-disease-patients-treated-with-lcig-analysis-from-the-cosmos-study/. Accessed December 17, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-on-dyskinesia-in-advanced-parkinsons-disease-patients-treated-with-lcig-analysis-from-the-cosmos-study/