Objective: To assess the tolerability of opicapone (OPC) when used in elderly patients with Parkinson’s disease (PD) in real-world conditions.
Background: OPC proved to be effective in the treatment of end-of-dose motor fluctuations in patients with PD [1,2]. The prevalence of PD increases with age owing to rising life expectancy in Western and many developing countries; the global burden of PD has more than doubled [3].
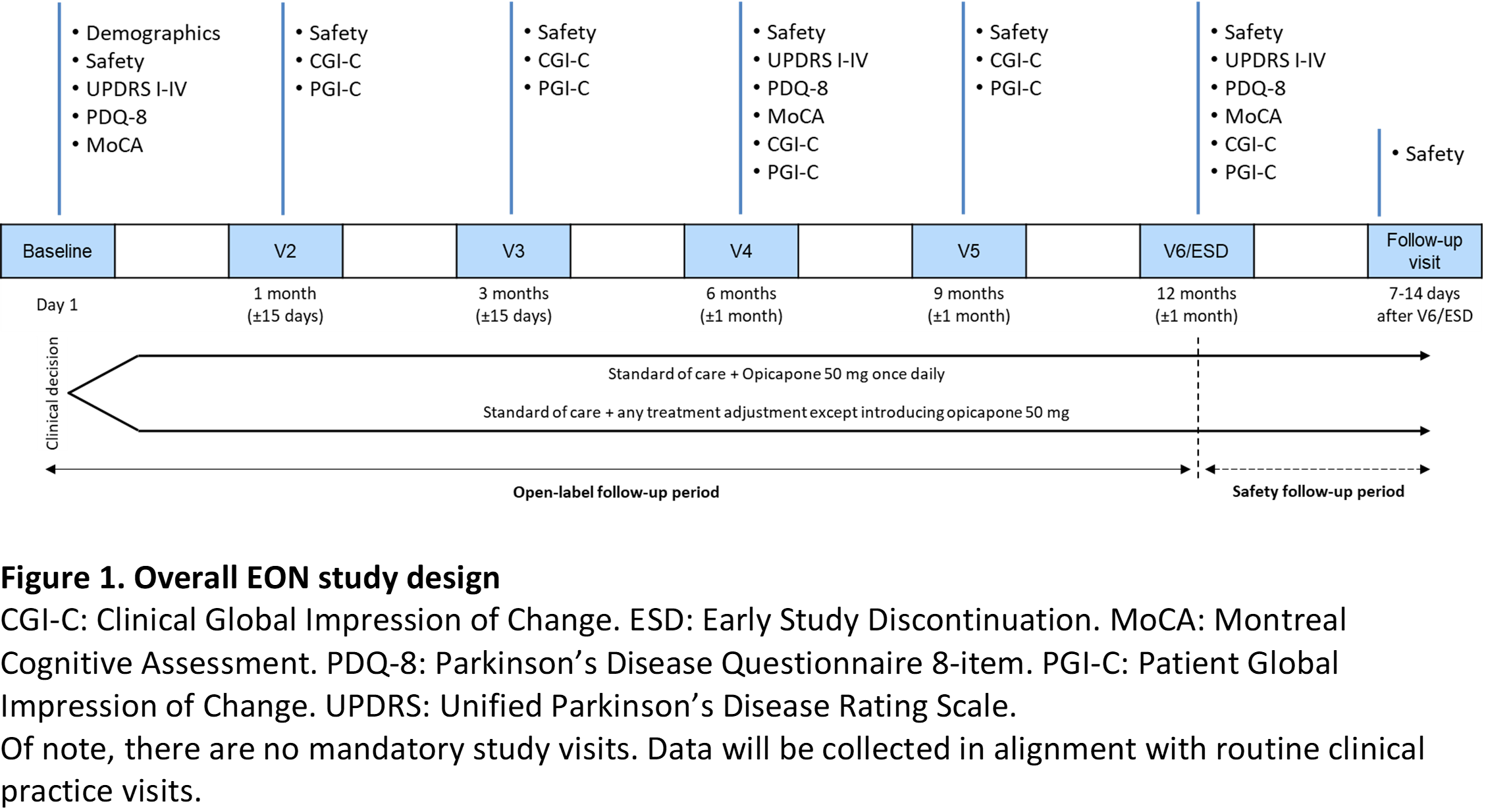
Method: Approximately 100 patients (aged ≥75 years) with idiopathic PD and wearing-off (end-of-dose deterioration) will receive OPC 50 mg once-daily or standard-of-care treatment during a 1-year follow-up period, according to the physician’s decision. There are no mandatory study visits. Data will be collected in alignment with routine clinical practice: at baseline (date of enrollment), 1, 3, 6, 9 and 12 months (Figure 1).
Results: The primary endpoint will be based on measures of tolerability. Secondary endpoints include functional motor and non-motor assessments (Unified Parkinson’s Disease Rating Scale [UPDRS], Montreal Cognitive Assessment [MoCA], Parkinson’s Disease Questionnaire-8 [PDQ-8]) and global impression of change scales (Clinical Global Impression of Change [CGI-C], Patient Global Impression of Change [PGI-C]). No formal sample size calculation was performed due to the exploratory nature of the study. First-patient-in occurred on 28/04/2020 and last-patient-out is expected for mid-2022, but timelines might be impacted by the COVID-19 pandemic situation. Currently, ≥20 sites in Germany, UK, Spain, Italy, and Portugal have actively recruited ≥20 patients.
Conclusion: This study will describe the tolerability of OPC 50 mg in elderly adults with PD in a real-world setting.
References: 1. Ferreira et al., Lancet Neurol. 2016;15(2):154-165 2. Lees et al., JAMA Neurol. 2017;74(2):197-206 3. GBD 2016. Lancet Neurol. 2018;17:939-53
To cite this abstract in AMA style:
V. Dostal, K. Chaudhuri, P. Odin, M. Kurtis, R. Costa, D. Magalhães, J. Rocha, P. Soares-da-Silva. The EON (Elderly patients on ONgentys) study in Parkinson’s disease: design and rationale of a prospective non-interventional trial [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-eon-elderly-patients-on-ongentys-study-in-parkinsons-disease-design-and-rationale-of-a-prospective-non-interventional-trial/. Accessed December 25, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-eon-elderly-patients-on-ongentys-study-in-parkinsons-disease-design-and-rationale-of-a-prospective-non-interventional-trial/