Category: Parkinson’s Disease: Clinical Trials
Objective: Utilize clinical trial data to analyze amantadine delayed release/extended release (AMT DR/ER) capsules as an early add-on for OFF and dyskinesia in people with Parkinson disease (PwPD).
Background: Bedtime-administered, AMT DR/ER capsules received FDA approval to treat levodopa-related dyskinesia in 2017.[1] The 2 pivotal trials supporting approval enrolled participants using levodopa for a mean 7.7 years, with over 77% using additional PD medications.[2] Based on significant reductions in OFF-time in both trials,[3][4] that appeared persistent though 2-years in an open-label trial,[5] the FDA granted AMT DR/ER an additional indication as a levodopa-adjunct for OFF episodes.
Wearing OFF can emerge within the first years of levodopa use; therefore, it’s important to know how AMT DR/ER performs as an early add-on to levodopa.
Method: Estimate efficacy of earlier (first add-on) AMT DR/ER use by evaluating PD diary data from pooled pivotal trials for 2 participant subgroups: 1) using levodopa monotherapy at baseline, or 2) ≤5 years since PD diagnosis at baseline.
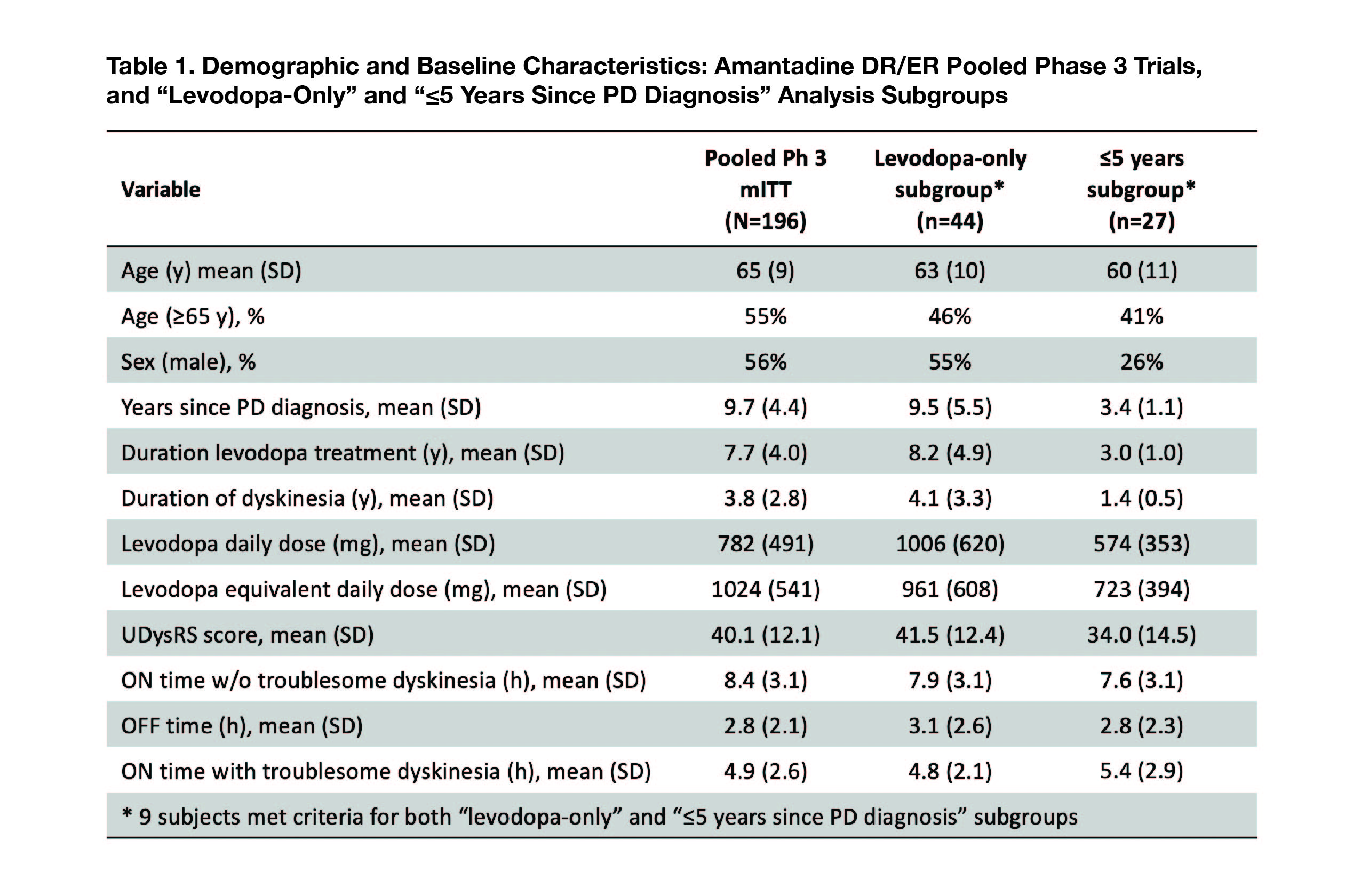
Results: Of 196 enrolled (mITT), 44 (22%; n= 28 AMT DR/ER; n= 16 placebo) composed the “levodopa-only” subgroup, and 27 (13.8%; n= 14; n=13) composed the “PD ≤5 years” subgroup. Nine patients were included in both subgroups. [Table 1]
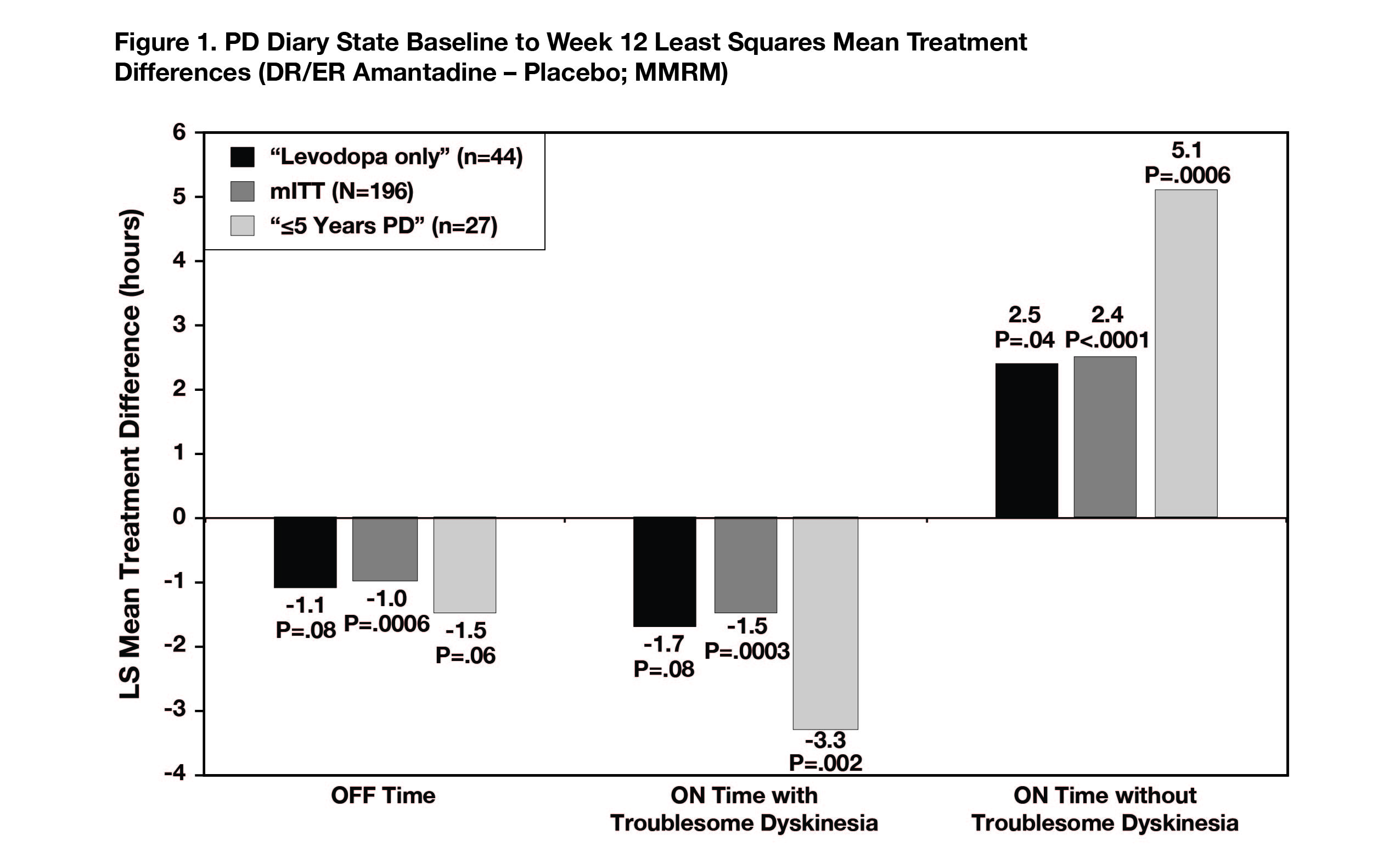
The levodopa-only subgroup had similar placebo-adjusted improvements in OFF time (-1.1 hours), ON time with troublesome dyskinesia (-1.7 hours), and ON time without troublesome dyskinesia (+2.4 hours) as overall phase 3 participants (-1.0, -1.5, +2.5 hours, respectively). The PD ≤5 years subgroup had numerically larger improvements in all three diary states (-1.5, -3.3, and +5.1 hours).[Figure 1]
Compared to overall trial results, the incidences of commonly occurring adverse events were (overall, levodopa-only, PD ≤5 years): hallucinations (21%, 21%, 29%), dizziness (16%, 21%, 21%), dry mouth (16%, 18%, 14%) peripheral edema (15%, 18%, 14%) constipation (13%, 11%, 21%), and fall (13%, 11%, 7%).
Conclusion: These post-hoc analyses, approximating AMT DR/ER efficacy as a first/early add-on to levodopa, showed OFF and dyskinesia reduction, and consequent ON time improvement without troublesome dyskinesia, that were similar to, or larger than the overall trial. Results suggest AMT DR/ER efficacy as a first add-on to levodopa for patients with early motor complications.
References: [1] Gocovri (amantadine) extended release capsules (prescribing information). Emeryville, CA: Adamas Pharmaceuticals, Inc.; 1/2020. [2] Elmer LW, Juncos JL, Singer C, et al. Pooled Analyses of Phase III Studies of ADS-5102 (Amantadine) Extended-Release Capsules for Dyskinesia in Parkinson’s Disease. CNS Drugs. Apr 2018;32(4):387-398. [3] Pahwa R, Tanner CM, Hauser RA, et al. ADS-5102 (Amantadine) Extended-Release Capsules for Levodopa-Induced Dyskinesia in Parkinson Disease (EASE LID Study): A Randomized Clinical Trial. JAMA Neurol. Aug 1 2017;74(8):941-949. [4] Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord. Dec 2017;32(12):1701-1709. [5] Tanner CM, Pahwa R, Hauser RA, et al. EASE LID 2: A 2-Year Open-Label Trial of Gocovri (Amantadine) Extended Release for Dyskinesia in Parkinson’s Disease. J Parkinsons Dis. Jan 6 2020.
To cite this abstract in AMA style:
C. Tanner, J. Lytle, A. Formella. Amantadine DR/ER Efficacy as Early Add-On for Motor Complications in Parkinson’s Disease [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/amantadine-dr-er-efficacy-as-early-add-on-for-motor-complications-in-parkinsons-disease/. Accessed December 23, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/amantadine-dr-er-efficacy-as-early-add-on-for-motor-complications-in-parkinsons-disease/