Category: Parkinson’s Disease: Clinical Trials
Objective: To describe blindness for treatment allocation in STEADY-PD3 and understand the reasons for perceived treatment allocation.
Background: The use of a placebo arm aims to control for the effect of expectation of benefit that is prevalent in PD. The preservation of blindness in a randomized control trial is thus essential for the accurate interpretation of clinical trial results.
Method: Exploratory analyses of STEADY-PD3 (NCT02168842). STEADY-PD3 enrolled 336 PD patients (<3 years of diagnosis, no dopaminergic medication) randomized to active treatment:placebo (1:1) and followed for 3 years. A blindness questionnaire (perceived treatment allocation and associated reasons) was completed by study participants and investigator at the end of treatment. We compared the adjusted mean change (multiple regression model) in the MDS-UPDRS and UPDRS total scores and subscales from baseline to year 3 between groups defined by perceived treatment allocation. We compared time-to-need for levodopa using a similar study group definition.
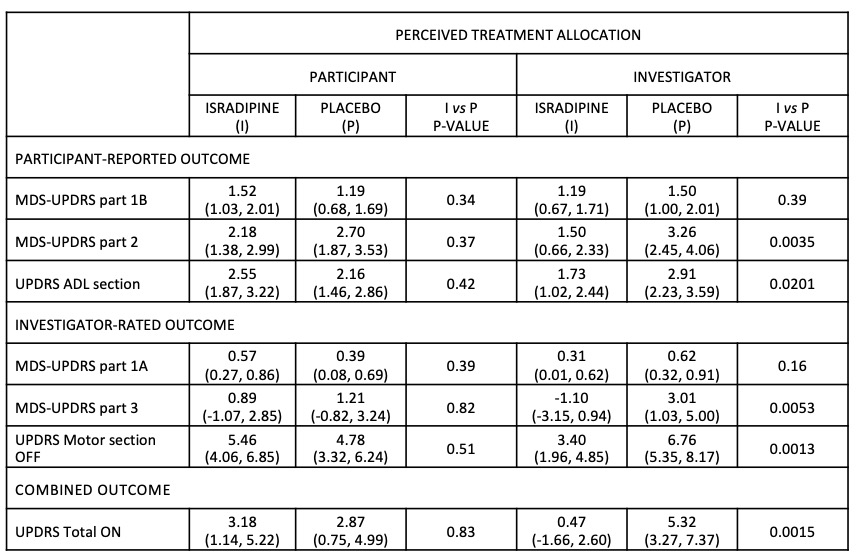
Results: 320 study participants completed the blindness questionnaire. Perceived allocation matched the real treatment allocation in 58% participants and 54% investigators. Main reasons for perceived allocation: [Isradipine] no disease progression (participants, n=34; investigator, n=60); symptomatic improvement (participants, n=41; investigator, n=33). [Placebo]: no symptomatic improvement (participants, n=70; investigator, n=64), no side effects (participants, n=40; investigator, n=81). There was a greater mean worsening in various efficacy outcomes (MDS-UPDRS part 2, p=0.0035; UPDRS ADL, p=0.0201, MDS-UPDRS part 3, p=0.0053; UPDRS Motor OFF, p=0.0013; Table 1) for a placebo vs. Isradipine-perceived allocation only according to investigator judgment and not when considering the participants’ perceived allocation. There were no other significant findings.
Conclusion: Investigator perceived allocation to placebo was associated with a faster progression in motor symptoms and ADLs impairment, but not for participant perceived allocation. The improvement of symptoms was one of the main reasons for perceived allocation by both participants and investigators, although a lack of symptomatic benefit of Isradipine was clearly communicated in the consent.
To cite this abstract in AMA style:
T. Mestre, S. Eberly, C. Grimes, D. Oakes, T. Simuni. Blindness analyses in STEADY-PD3: a phase III placebo-controlled double-blind randomized trial of Isradipine as a disease modifying agent in early Parkinson Disease [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/blindness-analyses-in-steady-pd3-a-phase-iii-placebo-controlled-double-blind-randomized-trial-of-isradipine-as-a-disease-modifying-agent-in-early-parkinson-disease/. Accessed January 1, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/blindness-analyses-in-steady-pd3-a-phase-iii-placebo-controlled-double-blind-randomized-trial-of-isradipine-as-a-disease-modifying-agent-in-early-parkinson-disease/