Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate dyskinesia and OFF interference with activities and experiences for persons with Parkinson disease (PWPD), and mitigation with treatment
Background: OFF and Dyskinesia remain significant sources of disability with levodopa treatment as Parkinson’s disease advances. Emphasis on avoiding the OFF state can lead to underestimating the impact of dyskinesia, rather than prioritizing strategies to mitigate both negative states. We evaluated the collective interference of dyskinesia and OFF across daily activities, and the ability of bedtime-administered delayed-release/extended release amantadine (AMT DR/ER),[1] the only medication with dual FDA-approval for OFF episodes and/or dyskinesia, to reduce this interference.
Method: Using pooled, phase-3 AMT DR/ER trials, [2] enrolling PWPD with dyskinesia (88% of whom also experienced OFF), we analyzed incremental number of items scored ≥2 (impacts performance) or ≥3 (substantial impact/results in avoidance) on each of 2 similarly-anchored, patient-rated, measures (UDysRS Part 1B and MDS-UPDRS Part II) of dyskinesia/motor symptom impact on daily activities and experiences. Treatment-related changes in cumulative items scored ≥2 or ≥3 were assessed using linear models with study and treatment effects.
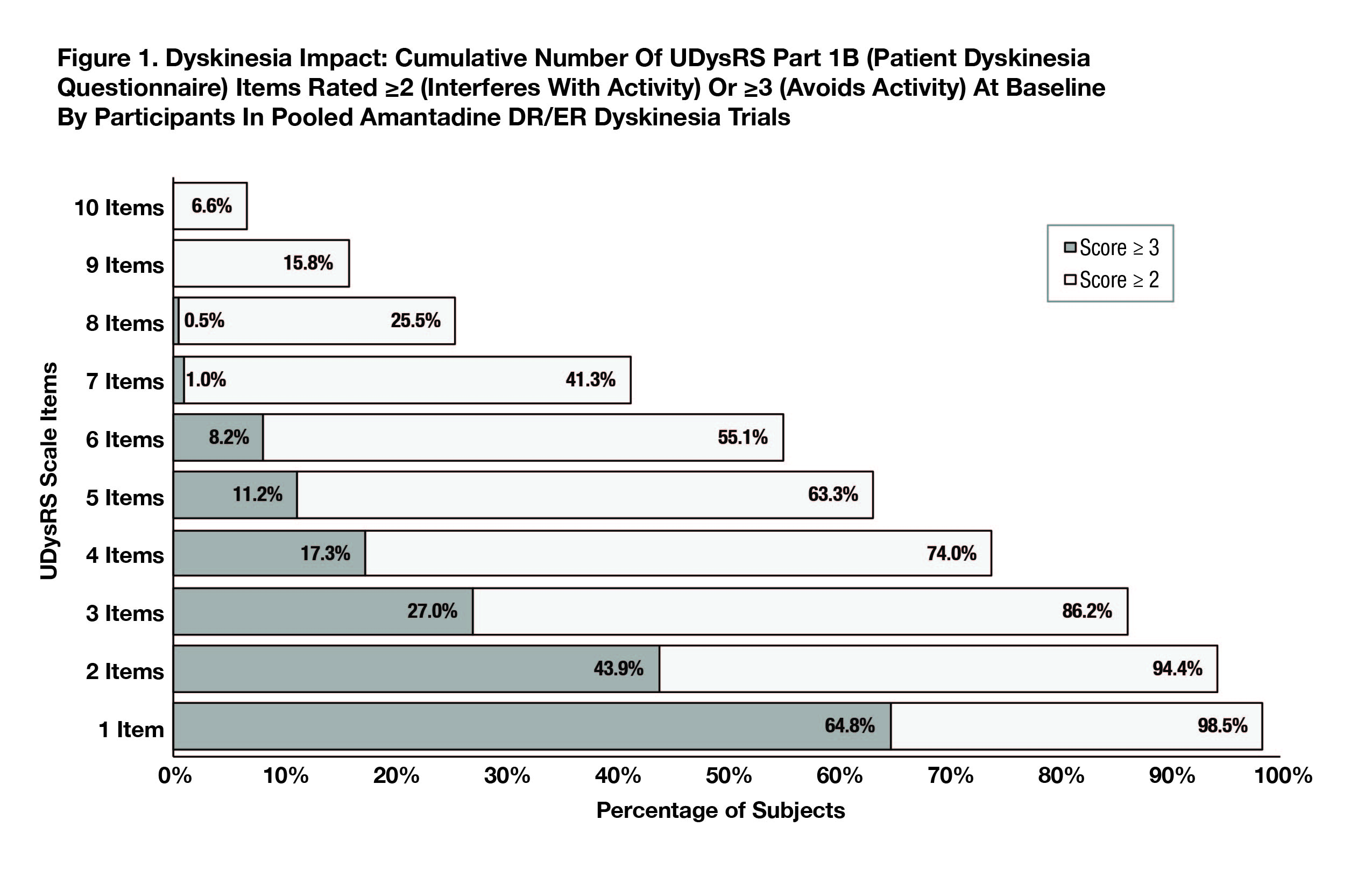
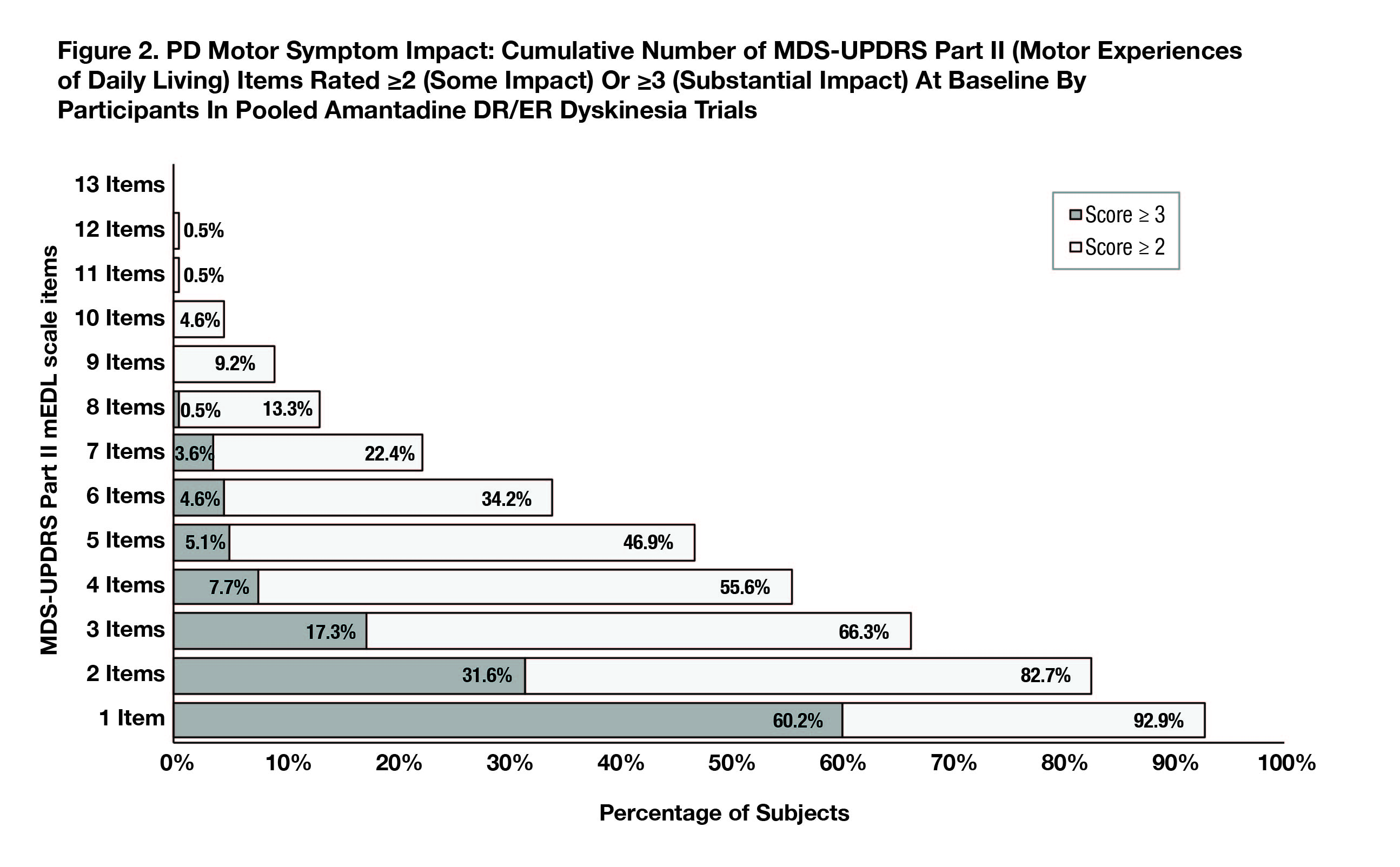
Results: At baseline (N=196; mITT) 98% (UDysRS) and 93% (MDS-UPDRS Part II) of participants reported interference/impact on 1 or more activities (score ≥2); for 65% and 60%, respectively, disability was substantial, or sufficient to result in activity avoidance (score ≥3).[Figure 1][Figure 2] Over half (55%) of PWPD reported dyskinesia interfered with ≥6 of 10 UDysRS items (7% noted interference with all 10) and 34% reported PD motor symptoms interfered with ≥6 of 13 MDS-UPDRS PII items.
AMT DR/ER significantly reduced dyskinesia and OFF and their associated impact on activities and experiences, with reduction in mean number of UDysRS items affected from [AMT DR/ER vs. Placebo] 5.5 vs. 5.8 at baseline to 2.2 vs. 3.7 at Week 12 (P<.01) and MDS-UPDRS Part II items affected from 4.3 vs. 4.3 at baseline to 2.7 vs. 3.8 at week 12 (P<.01).
Conclusion: Dyskinesia and OFF impact multiple areas of function. AMT DR/ER-related dyskinesia and OFF reduction were accompanied by reductions in cumulative number of UDysRS Part 1B and MDS-UPDRS Part II items rated as causing meaningful burden on the lives of PWPD.
References: [1] Gocovri (amantadine) extended release capsules (prescribing information). Emeryville, CA: Adamas Pharma, LLC; 1/2020 [2] Elmer LW, Juncos JL, Singer C, et al. Pooled Analyses of Phase III Studies of ADS-5102 (Amantadine) Extended-Release Capsules for Dyskinesia in Parkinson’s Disease. CNS Drugs. Apr 2018;32(4):387-398.
To cite this abstract in AMA style:
K. Lyons, R. Pahwa, N. Crouse, L. Llorens, R. Howard, A. Formella. Amantadine DR/ER-Related Reduction in OFF and Dyskinesia Improved Patient-Rated Interference with Activities and Social Interactions [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/amantadine-dr-er-related-reduction-in-off-and-dyskinesia-improved-patient-rated-interference-with-activities-and-social-interactions/. Accessed October 17, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/amantadine-dr-er-related-reduction-in-off-and-dyskinesia-improved-patient-rated-interference-with-activities-and-social-interactions/