Category: Parkinson’s Disease: Clinical Trials
Objective: To investigate satisfaction with daily remote monitoring with a digital health technology tool (DHTT) in individuals with early Parkinson’s disease (PD) and the relationship with DHTT adherence and motor disease severity.
Background: DHTTs assess motor signs of PD objectively, reliably, and frequently. Patients’ satisfaction with the DHTT and testing schedule may inform data interpretation and future DHTT development.
Method: A total of 316 individuals with early-stage PD in the PASADENA Phase II study (NCT03100149) were remotely monitored over two years with the Roche PD Mobile Application v2, 53% (n=169) of whom completed an end-of-study DHTT satisfaction questionnaire at the timepoint of the present analyses. They were demographically and clinically comparable to the remaining population. Roche’s DHTT comprised ten active tests (half performed daily) and surveys on smartphones plus passive monitoring via smartwatch and -phone. The questionnaire had eight 5-point Likert questions on acceptance of different DHTT facets (1=very negative, 3=neutral, 5=very positive), a question on the least preferred component and open feedback. Overall satisfaction was defined as the mean Likert score, which was then correlated with adherence and motor sign severity (MDS-UPDRS Part III).
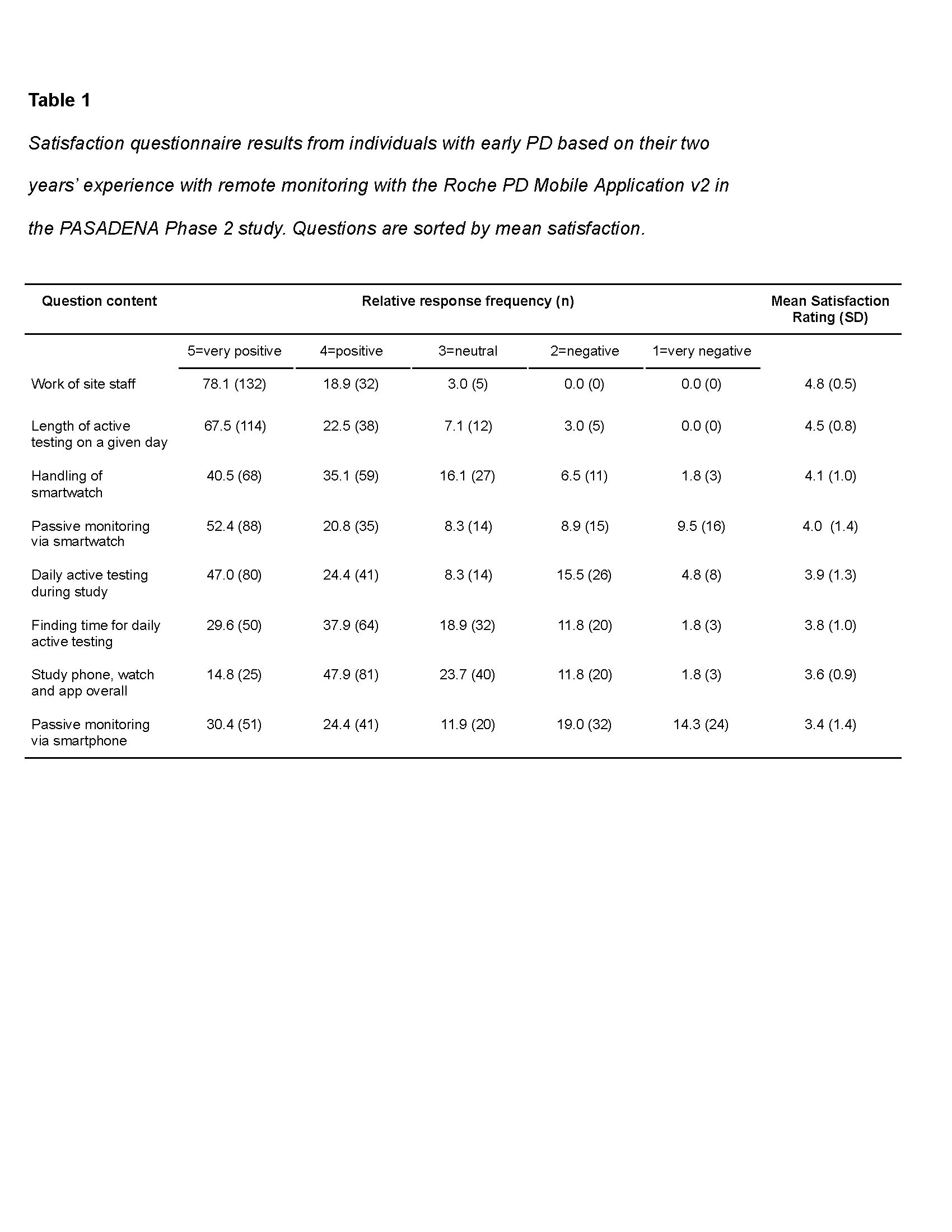
Results: Overall satisfaction was high (M=4.0, SD=0.7), with the majority of patients positively rating: length of active testing on a given day (90% pos., 3% neg), daily active testing during study (71% pos. 20% neg) and passive monitoring via smartwatch (73% pos., 18% neg.) and -phone (55% pos. 33% neg.) [table1]. The least preferred components were surveys (33%) and passive monitoring with provisioned devices (37%). Longer battery life and device aesthetics were noted as areas for future improvement in the open-feedback section. Overall satisfaction correlated with adherence (r=.42, p<.001) but not with MDS-UPDRS Part III scores (r=.08, p=.28).
Conclusion: Overall acceptance of daily testing over two years in the Phase II PASADENA study was high. Satisfaction correlated with adherence, but not with motor disease severity. Improvements in battery life and design of the provisioned devices could further increase satisfaction and adherence. Optimization of DHTT satisfaction with patient input is critical to minimize patient burden and ensure high quality digital outcome measures.
To cite this abstract in AMA style:
B. Fehlmann, A. Thomann, W. Popp, F. Lipsmeier, E. Volkova-Volkmar, J. Friess, H. Svoboda, G. Pagano, T. Kilchemann, A. Bamdadian, W. Zago, R. Postuma, M. Lindemann, K. Taylor. Relationship of satisfaction and adherence in remote digital monitoring: Results from a clinical drug trial in early Parkinson’s disease [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/relationship-of-satisfaction-and-adherence-in-remote-digital-monitoring-results-from-a-clinical-drug-trial-in-early-parkinsons-disease/. Accessed December 28, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/relationship-of-satisfaction-and-adherence-in-remote-digital-monitoring-results-from-a-clinical-drug-trial-in-early-parkinsons-disease/