Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the efficacy and safety of Tuina treatment in treating non-motor symptoms (NMS) of Parkinson’s disease (PD) and provide preliminary data for conducting future full-scale clinical studies.
Background: PD is the second most prevalent primary neurodegenerative disease. Its motor symptoms (MS) and NMS cause a heavy burden on patients and caregivers. There is presently no cure to PD but symptomatic therapies e.g. levo-dopa. Despite their high effectiveness in treating MS, prolonged use invokes complications and worsens NMS. Effective treatments for NMS, which highly interrupts patients’ daily life and health, are yet to be discovered. Tuina in TCM, a form of Chinese manipulative therapy practiced by ancient TCM doctors to cure diseases and promote health can be a potential approach.
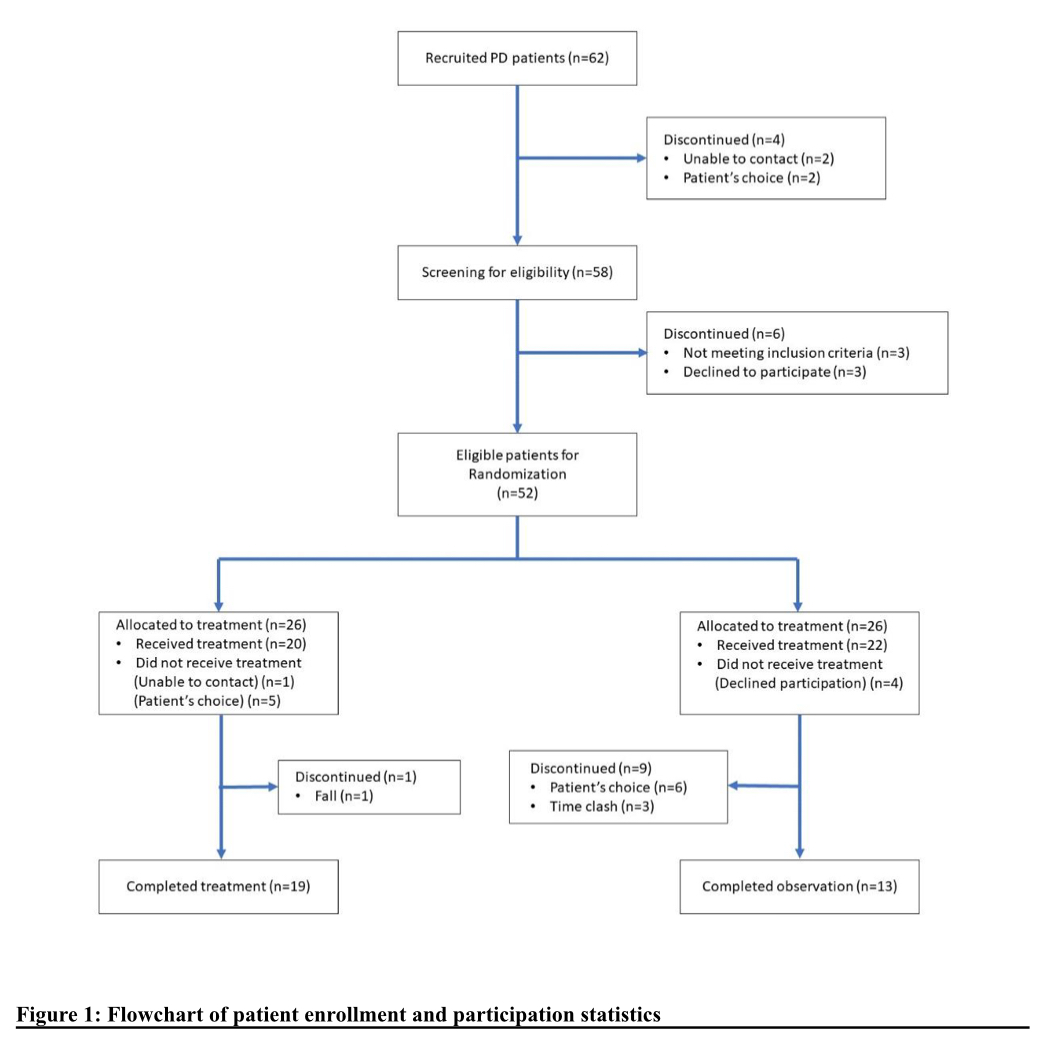
Method: This is a rigorously conducted assessor-and-data-analyzer-blind clinical study, conforming to the guidelines of Consolidated Standards of Reporting Trials (CONSORT). 52 eligible patients were randomly allocated into the treatment group for Tuina or control group without any extra intervention aside from conventional treatment (1:1) [figure1]. Assessments were done during the 8-week treatment period to observe for any change. The primary outcome is the difference in total and domain scores of the English version of Non-motor symptoms scale (NMSS) between week 8 and baseline. The secondary outcomes are the total scores of a customized Chinese version of Parkinson’s Disease Questionnaire (C-PDQ-16) and the Deficiency of Spleen Qi Questionnaire Scoring (DSQQ).
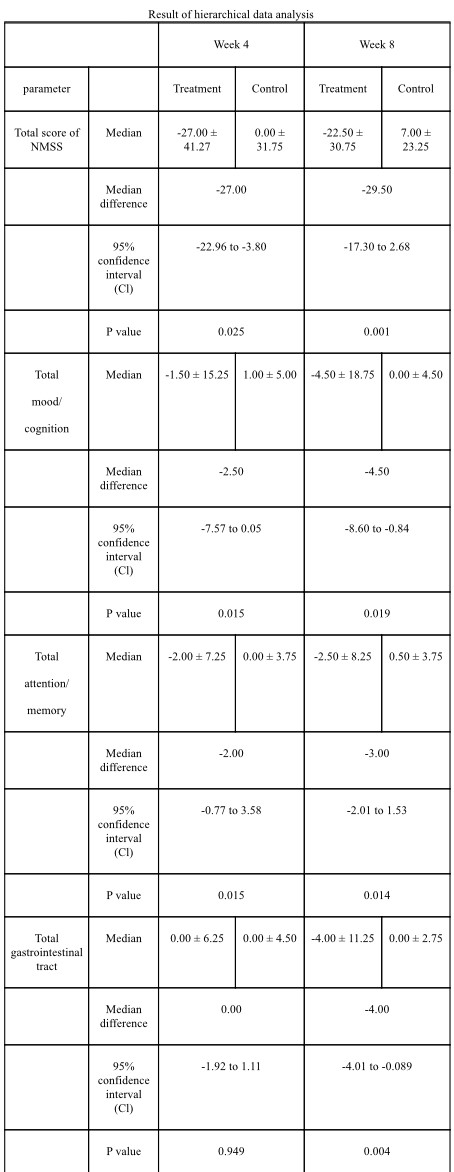
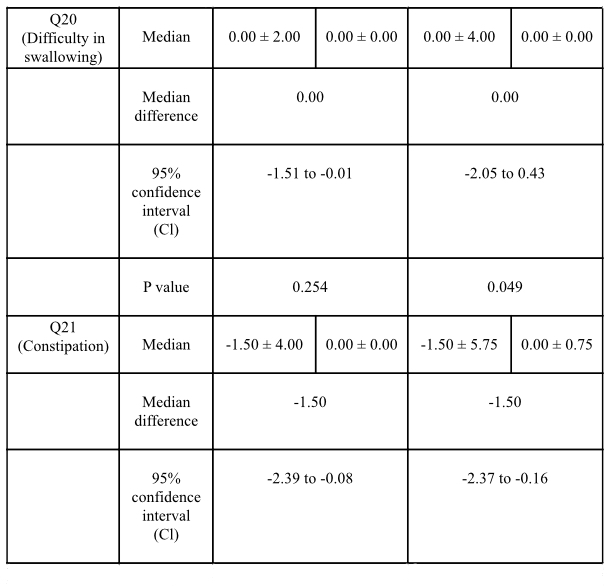
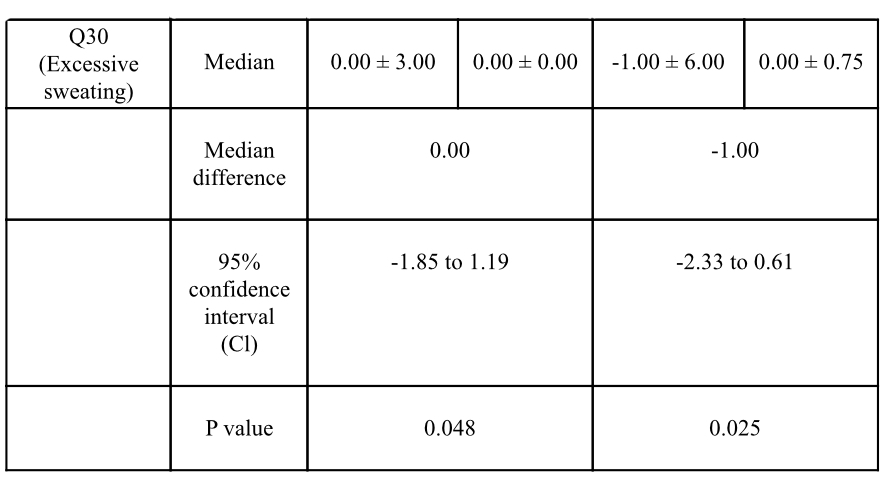
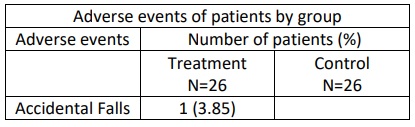
Results: Data analysis revealed statistically significant improvement in total and domain scores of NMSS, including scores of 4 areas: total score (p=0.001), mood/cognition (p=0.019), attention/memory (p=0.014) and GI tract (p=0.004) [table1]. Post-hoc analysis showed statistically significant improvement in constipation (p=0.005), difficulty in swallowing (p=0.049) [table 2] and excessive sweating (p=0.025) [table3]. C-PDQ-16 daily life domain (p=0.032) and DSQQ total score showed improvement by the end of the treatment period. No severe adverse events were reported in the treatment group regarding its safety [table4].
Conclusion: Tuina in TCM is safe. It showed some improvement in treating NMS of PD. A further full-scale clinical trial of Tuina in TCM is warranted to support its finding.
To cite this abstract in AMA style:
FY. Chung, HY. Lai, CS. Yuen, HW. Lam, KY. Lai, SM. Chan, M. Li, KK. Chua. The effect of Tuina in Traditional Chinese Medicine in treatment of Parkinson’s disease (Non-motor symptoms): a pilot clinical study [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-effect-of-tuina-in-traditional-chinese-medicine-in-treatment-of-parkinsons-disease-non-motor-symptoms-a-pilot-clinical-study/. Accessed December 23, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-effect-of-tuina-in-traditional-chinese-medicine-in-treatment-of-parkinsons-disease-non-motor-symptoms-a-pilot-clinical-study/