Category: Parkinson’s Disease: Clinical Trials
Objective: This post-hoc analysis evaluated the impact of 3-month earlier versus postponed initiation of opicapone (OPC) in patients with Parkinson’s disease (PD) and motor fluctuations.
Background: OPC proved to be effective in treating end-of-dose motor fluctuations in patients with PD [1,2].
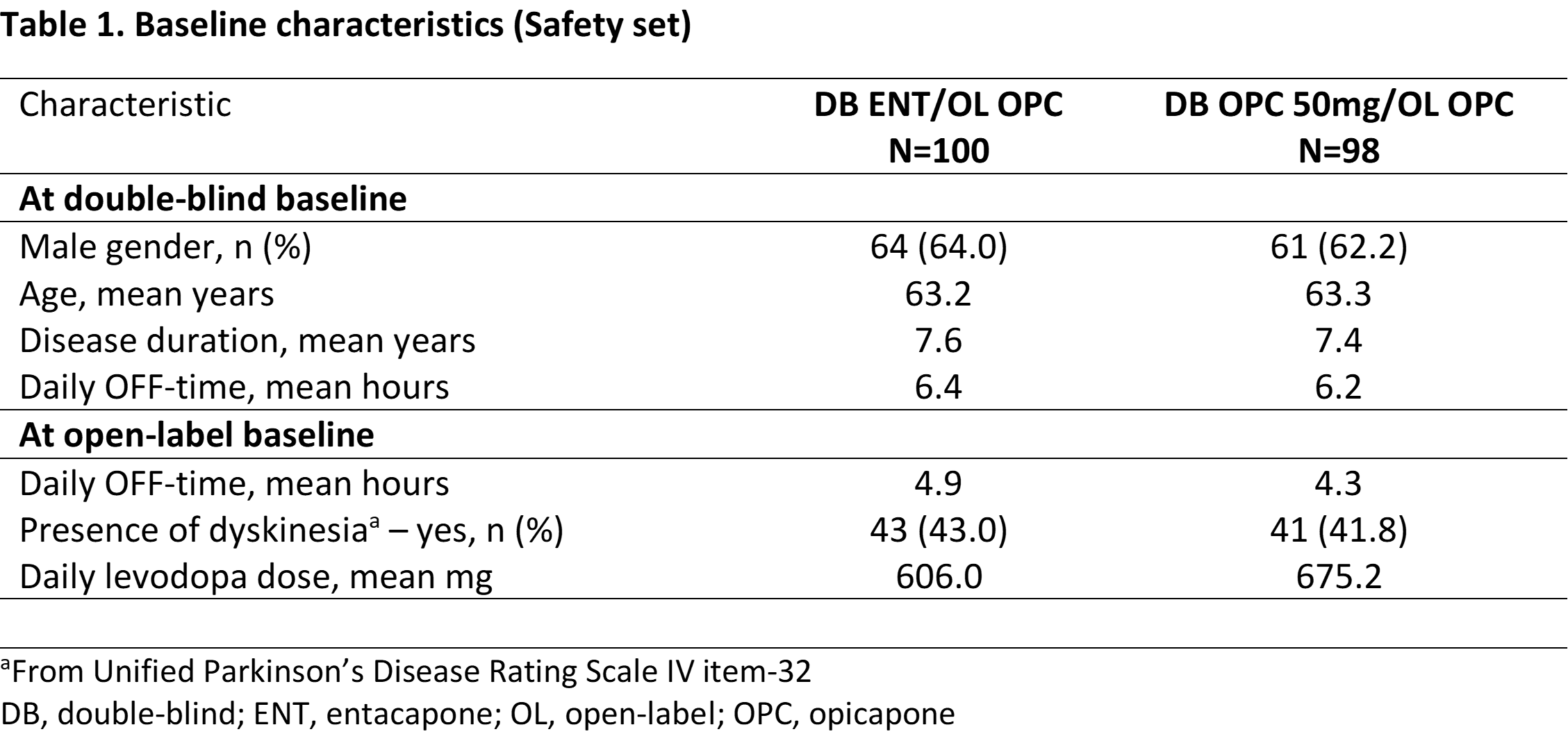
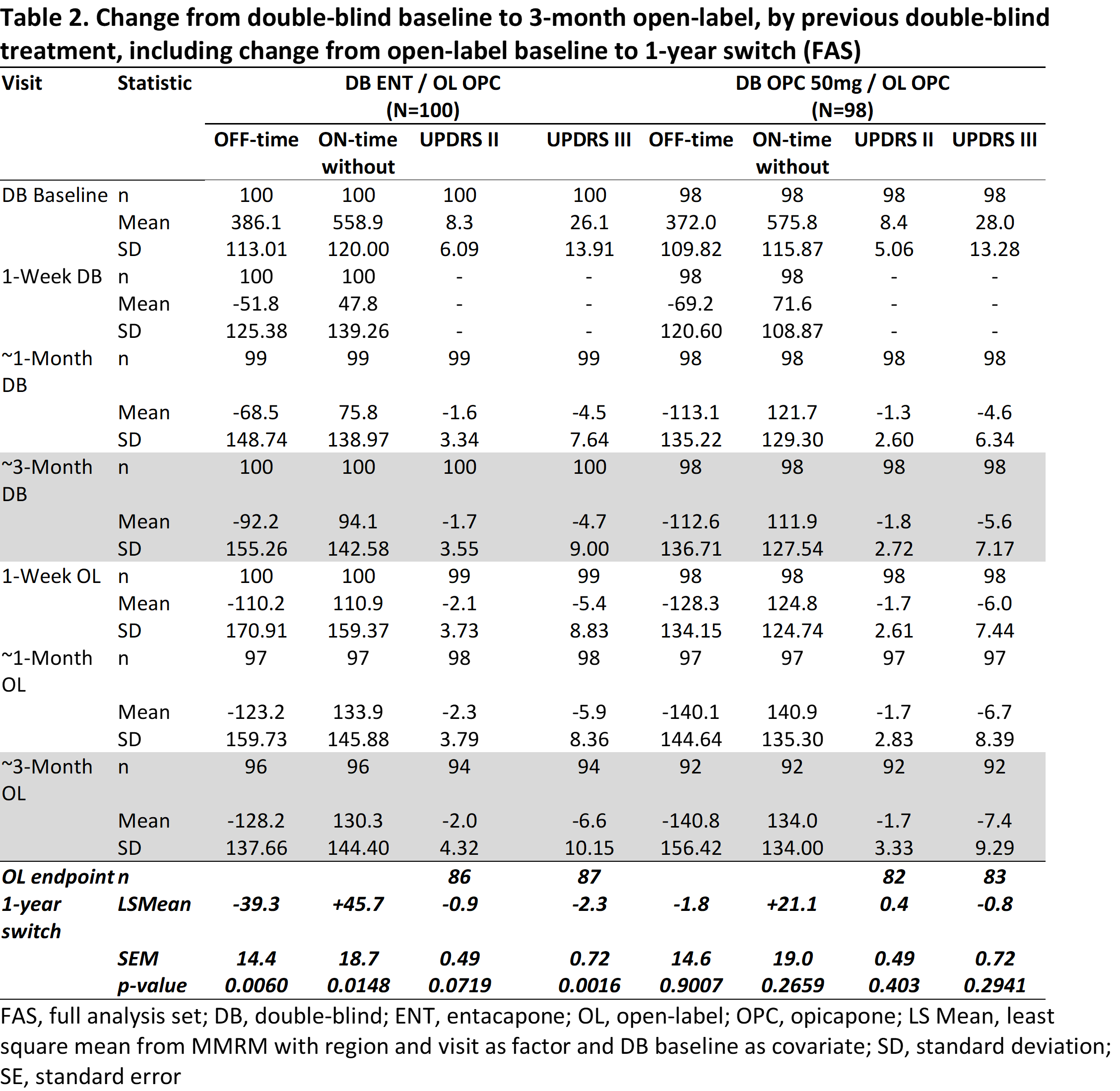
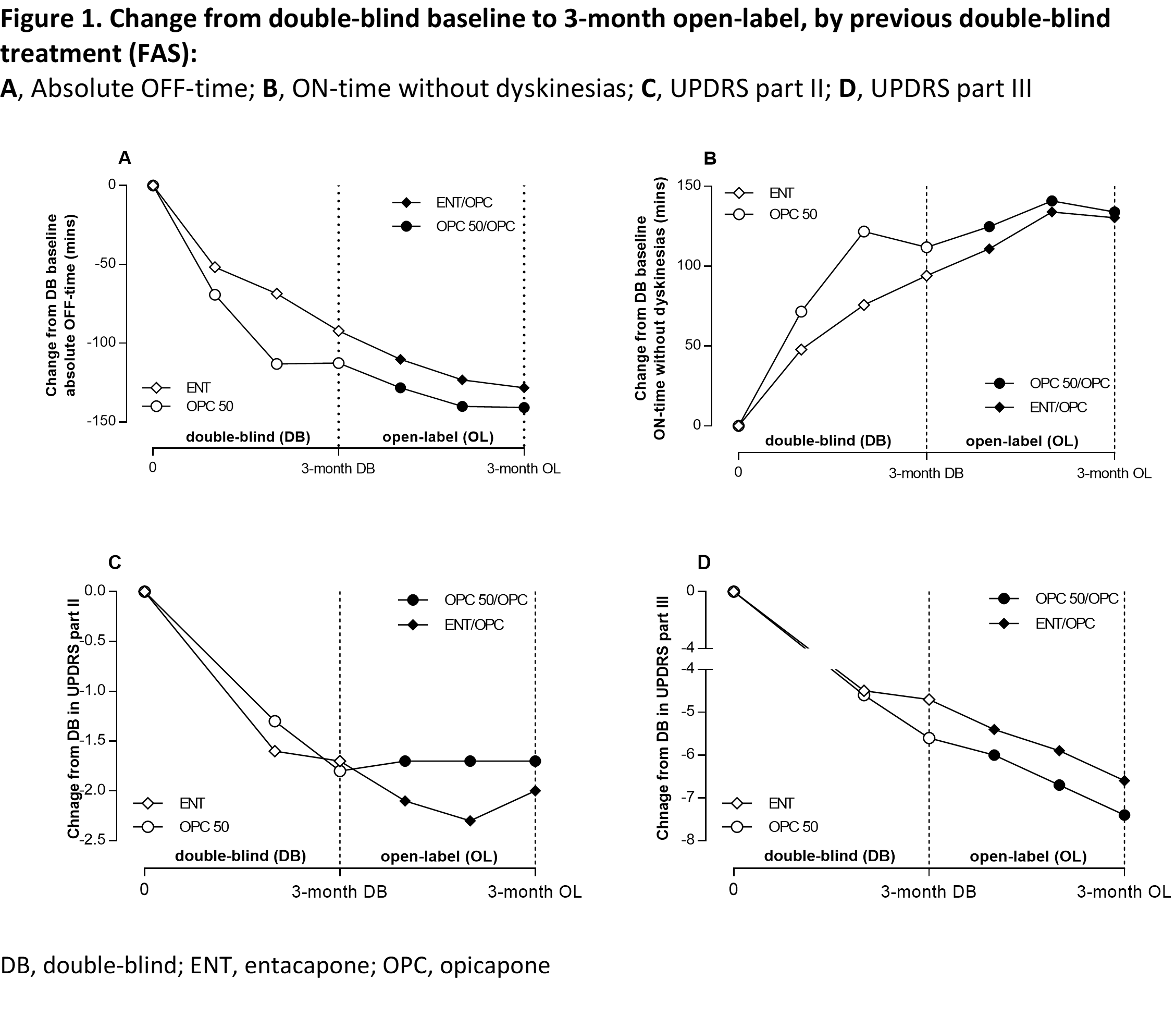
Method: OPC 50 mg and entacapone (ENT) data from the BIPARK-I study [1] were analyzed. The primary efficacy endpoint was change from baseline in absolute OFF-time. Secondary efficacy outcomes included ON-time without-dyskinesia, Unified-PD-Rating-Scale (UPDRS) part-II and III. Patients receiving levodopa/dopa-decarboxylase inhibitor (DDCi) were randomized to OPC 50 mg (‘early-start’: 50 mg-OPC) or ENT (‘postponed-start’: ENT-OPC) for a 3-month, double-blind phase, after which all patients received open-label levodopa/DDCi and OPC for up to 1 year. This post-hoc analysis evaluated the impact of 3-month earlier versus postponed initiation of OPC 50 mg by comparing 3-month initial treatment with both OPC 50 mg and ENT and a 3-month switch cut-off under open-label OPC treatment.
Results: In total, 198 patients switched from OPC 50 mg (n=98) or ENT (n=100) to 1-year OPC open-label extension. At the 3-month double-blind endpoint, OPC 50 mg showed greater reduction in OFF-time, UPDRS-II and III, and ON-time without-dyskinesia increase. At the 3-month open-label cut-off, OPC 50 mg-OPC ‘early-start’ showed greater reduction in OFF-time, UPDRS-III, and ON-time without-dyskinesia increase. Furthermore, at the 1-year open-label endpoint, a statistically significant benefit for ENT-OPC postponed initiation (even as short as 3 months) was still observed, except for UPDRS part II. Both OPC 50 mg and ENT groups were well tolerated.
Conclusion: These data suggest that early rather than delayed (or second-line) addition of OPC 50 mg to levodopa/DDCI provides an extended benefit over ENT.
References: 1. Ferreira JJ, et al. Lancet Neurol. 2016;15:154–65. 2. Lees AJ, et al. JAMA Neurol. 2017;74:197–206.
To cite this abstract in AMA style:
C. Carroll, A. Lees, J. Ferreira, M. Fonseca, D. Magalhaes, J. Rocha, P. Soares-da-Silva. Impact of 3-month earlier versus postponed initiation of opicapone versus entacapone in levodopa-treated patients with Parkinson’s disease and motor fluctuations [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/impact-of-3-month-earlier-versus-postponed-initiation-of-opicapone-versus-entacapone-in-levodopa-treated-patients-with-parkinsons-disease-and-motor-fluctuations/. Accessed December 31, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-of-3-month-earlier-versus-postponed-initiation-of-opicapone-versus-entacapone-in-levodopa-treated-patients-with-parkinsons-disease-and-motor-fluctuations/