Category: Huntington's Disease
Objective: To summarize clinical experience and outcomes with deutetrabenazine (DTBZ) as a treatment for Huntington’s disease (HD) chorea from the Huntington’s Disease Society of America (HDSA) Center of Excellence (COE) at the University of Alabama at Birmingham (UAB).
Background: In 2017, DTBZ—a deuterated form of tetrabenazine (TBZ)—became the second FDA-approved medication to treat HD chorea in the US.1 In the original pivotal trial, DTBZ was found to have efficacy similar to TBZ but had a more favorable tolerability profile.1 Real world data on use of DTBZ since approval has been limited. Here, we examine our site’s cumulative clinical experience with DTBZ.
Method: We performed a retrospective chart review of all patients who initiated treatment with DTBZ between January 2017 and May 2019 in the HDSA COE at UAB. Patients with other movement disorders were excluded from analysis, and information on dosing, total maximal chorea (TMC) scores, subjective reports of efficacy, and adverse events (AE) were collected.
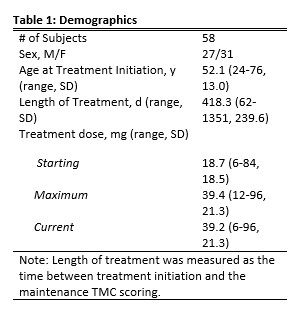
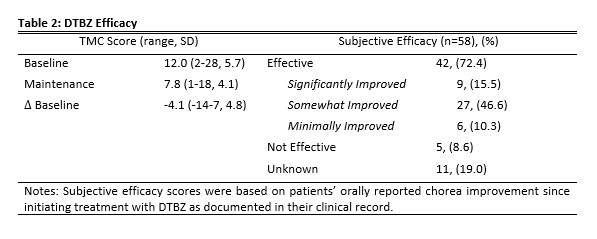
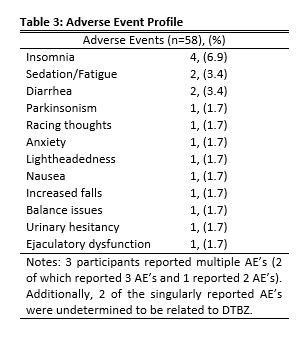
Results: The study population consisted of 58 patients (27 males [46.6%]) (Table 1). The mean treatment difference was a 4.1 (-14-7, 4.8) point decrease in TMC between baseline and maintenance visits (Table 2). Subjective efficacy reporting by patients showed that 42 patients (72.4%) described their treatment with DTBZ as “effective” (Table 2). Only 5 patients (8.6%) described their treatment with DTBZ as “not effective” with their chorea either being unchanged or worse (Table 2). Only 12 patients (20.7%) reported any AE, with 3 patients (5.2%) experiencing more than one AE. The most common AE was insomnia (6.9%) (Table 3). Additional AE’s include somnolence and diarrhea (3.4% each) (Table 3). The mean maintenance dose was 39.2 mg (6-96, 21.3) (Table 1).
Conclusion: Overall our real-world clinical experience with DTBZ shows good efficacy for the treatment of HD chorea and a low rate of AE’s. These data support the efficacy and tolerability seen in the original pivotal, with a relatively large sample size. Future studies should look to repeat this on a larger scale and across greater geography and practice pattern.
References: 1. Huntington Study G, Frank S, Testa CM, et al. Effect of Deutetrabenazine on Chorea Among Patients With Huntington Disease: A Randomized Clinical Trial. JAMA 2016;316:40-50.
To cite this abstract in AMA style:
K. Curtis, B. Denson, V. Sung. Cumulative Real-World Experience with Deutetrabenazine for Huntington’s Disease Chorea at a Single Center of Excellence in the US [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/cumulative-real-world-experience-with-deutetrabenazine-for-huntingtons-disease-chorea-at-a-single-center-of-excellence-in-the-us/. Accessed February 16, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/cumulative-real-world-experience-with-deutetrabenazine-for-huntingtons-disease-chorea-at-a-single-center-of-excellence-in-the-us/