Objective: To assess the efficacy and safety of incobotulinumtoxinA for lower-limb (LL) and upper-limb (UL) spasticity in children and adolescents with cerebral palsy (CP) and Gross Motor Function Classification System (GMFCS) levels IV–V using pooled data from the first injection cycle in two randomized Phase 3 studies, TIM (NCT01893411) and XARA (NCT02002884).
Background: Patients with CP have a wide range of motor impairments with a substantial proportion showing severe impairment (GMFCS level IV or V).
Method: Non-ambulant patients with GMFCS levels IV–V were analyzed (2–17 years of age with uni-/bilateral CP and Ashworth Scale [AS] score ≥2 in clinical patterns for treatment). Patients were randomized to three incobotulinumtoxinA dose groups: 8, 6, 2 U/kg body weight, maximum 200, 150, 50 U, per LL clinical pattern in TIM, and per UL in XARA. Additional multipattern treatment was allowed in both studies with total body doses up to 16 U/kg (≤400 U). Changes from baseline in AS and Global Impression of Change Scale (GICS) scores at Week 4 were assessed in those who had LL treatment (TIM and XARA) and those who had UL treatment (XARA). The incidence of adverse events (AEs) was also assessed.
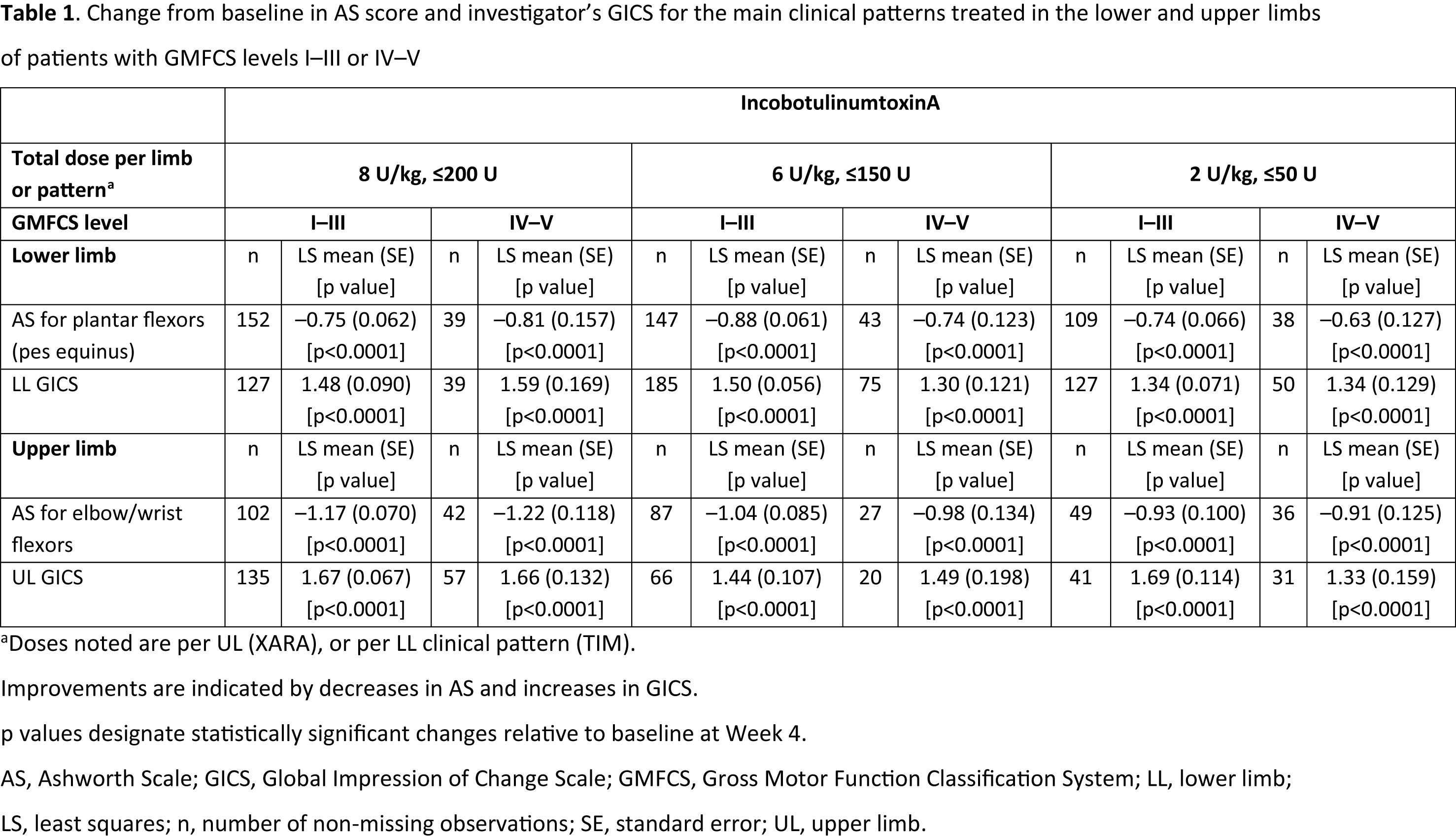
Results: Of patients with GMFCS levels IV–V, 164 received LL treatment and 108 received UL treatment. Statistically significant improvements in AS score for the pes equinus and flexed elbow/wrist and investigator’s GICS for both the LL and UL were seen with all incobotulinumtoxinA doses at Week 4 in patients with GMFCS levels IV–V and I–III (p<0.0001 vs baseline [table1]). In patients with GMFCS levels IV–V, AS and GICS improvements were numerically greatest in the high-dose group. The frequency of AEs was generally <30.0% across dose groups and GMFCS levels I–V. Fewer patients with GMFCS levels IV–V (21.4%) vs I–III (25.7%) experienced AEs. Few AEs of special interest (IV–V: 1.1%; I–III: 1.3%), treatment-related AEs (IV–V: 0.5%; I–III: 2.5%), or serious AEs (IV–V: 1.6%; I–III: 1.9%) occurred.
Conclusion: IncobotulinumtoxinA was effective, safe, and well-tolerated for multipattern treatment of LL and UL spasticity in children with severe CP (GMFCS levels IV–V).
Presented at TOXINS 2021 (Jan 16–17).
To cite this abstract in AMA style:
P. Kaňovský, D. Gaebler-Spira, A. Schroeder, H. Chambers, E. Dabrowski, T. Geister, A. Hanschmann, I. Pulte, M. Althaus, M. Banach, F. Heinen. Pooled efficacy and safety analysis of incobotulinumtoxinA for upper- and lower-limb spasticity in children with severe cerebral palsy (GMFCS levels IV–V) [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/pooled-efficacy-and-safety-analysis-of-incobotulinumtoxina-for-upper-and-lower-limb-spasticity-in-children-with-severe-cerebral-palsy-gmfcs-levels-iv-v/. Accessed December 26, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/pooled-efficacy-and-safety-analysis-of-incobotulinumtoxina-for-upper-and-lower-limb-spasticity-in-children-with-severe-cerebral-palsy-gmfcs-levels-iv-v/