Session Information
Date: Thursday, June 23, 2016
Session Title: Parkinson's disease: Clinical trials, pharmacology and treatment
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To evaluate the duration of treatment (DOT) and safety of apomorphine continuous subcutaneous infusion (CSI) in patients with advanced PD inscribed in a managed care program.
Background: Apomorphine CSI is one of the established advanced therapies for PD patients. Starting in 2010, patients were offered participation in a managed care program providing patient and caregiver training for CSI, pumping device instructions and continued ready-mixed apomorphine solution supply. Patients gave informed consent to collect and to process basic demographic and medication related safety data. In March 2015 the training program was changed and intensified.
Methods: We conducted a retrospective evaluation of the managed care program derived data from 2010 to February 2015 to determine the DOT with s. c. apomorphine using a Kaplan-Meier estimation. Safety data were reported by standard pharmacovigilance procedures to the marketing authorization holder of apomorphine. The safety database was related to the managed care program database to determine the number and severity of adverse events (AE’s) in the observed population. AE’s were standardized to the drug exposition in “patient years”. Subgroup analyses for patients with shorter (≤ 254 days) and longer DOT (> 254 days) were performed.
Results: 263 patients (m/f: 146/117, median age 70 years, range: 34 to 86 years) were enrolled into the managed care program. Median (mean) DOT was 458 (702) days (range from 1 to 1.699 days). 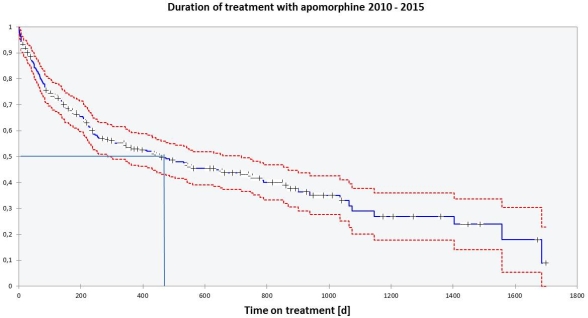 The Kaplan-Meier plot shows a steep slope in the early treatment period reflecting a high patient drop-out rate. Patient exposure to apomorphine was 301.6 patient years. The total number of reported AE’s was 407 equivalent to 1.3 AE’s per patient year. 151 patients stopped treatment for any reason. In 120 cases the reason for stopping was documented and showed the following distribution: change of therapy (40.8 %), intolerance (18.3 %), death (14.2 %), loss of efficacy (10.8 %), patient request (3.3 %), not otherwise specified (12.5 %).
The Kaplan-Meier plot shows a steep slope in the early treatment period reflecting a high patient drop-out rate. Patient exposure to apomorphine was 301.6 patient years. The total number of reported AE’s was 407 equivalent to 1.3 AE’s per patient year. 151 patients stopped treatment for any reason. In 120 cases the reason for stopping was documented and showed the following distribution: change of therapy (40.8 %), intolerance (18.3 %), death (14.2 %), loss of efficacy (10.8 %), patient request (3.3 %), not otherwise specified (12.5 %).
Conclusions: In this real-life population of patients with advanced PD under apomorphine treatment we demonstrated good tolerability with an expected safety profile for subcutaneous apomorphine therapy. Early drop-outs are frequently seen. The adherence seemed to improve with increasing treatment duration. More intensified patient support might change this finding.
To cite this abstract in AMA style:
D. Maessen, G. Ebersbach, L. Timmermann, A. Ceballos-Baumann. Apomorphine treatment in advanced Parkinson’s disease (PD): Duration of treatment and safety experience in a managed care program in Germany [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/apomorphine-treatment-in-advanced-parkinsons-disease-pd-duration-of-treatment-and-safety-experience-in-a-managed-care-program-in-germany/. Accessed April 3, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/apomorphine-treatment-in-advanced-parkinsons-disease-pd-duration-of-treatment-and-safety-experience-in-a-managed-care-program-in-germany/
