Session Information
Date: Thursday, June 23, 2016
Session Title: Parkinson's disease: Clinical trials, pharmacology and treatment
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To evaluate the effect of levodopa-carbidopa intestinal gel (LCIG, designated in the United States as carbidopa-levodopa enteral suspension) on motor fluctuations and dyskinesia in advanced Parkinson’s disease (PD) patients during routine care.
Background: LCIG is continuously delivered via percutaneous gastrojejunostomy (PEG-J) in advanced PD patients with motor fluctuations and dyskinesia not adequately reduced by available oral anti-Parkinsonian medications. LCIG treatment significantly improved motor complications and dyskinesia in this registry at interim follow-up (12 months [M]). (ref 1)
Methods: GLORIA is a multinational (18 countries, 75 centres) registry designed to collect efficacy and safety data of routine clinical care use of LCIG over 24M via PEG-J. Daily hours of “off” time and “on” time with dyskinesia were measured by modified Unified Parkinson’s disease Rating Scale Part IV items 39 and 32, respectively. Adverse drug reactions (ADRs) were monitored.
Results: Of the 375 patients enrolled (Table 1), 225 (60%) were LCIG-naïve and 150 (40%) were treated with LCIG for ≥12M before the registry; 258 (69%) completed the 24M follow-up. The mean (SD) levodopa dose was 1412 (606) mg on D1 [n=348] and 1512 (646) mL at M24 [n=257]. The percentage of patients taking concomitant medication (Table 2) ranged 60 – 64% (190/316, 175/273, respectively). LCIG-treated patients had significant decreases from baseline in daily hours of “off” time and “on” time with dyskinesia, which were maintained over 24M.(Figure 1) Forty-six patients (12%) discontinued because of an ADR; device dislocation was the only reason reported by ≥2 patients (0.2%).
| Demographic or Characteristic | n (% of N=375) | Mean (SD) | |
| Gender | Male | 220 (59) | |
| Female | 155 (41) | ||
| Age, years | 66.4 (8.8) | ||
| Race | White | 361 (98) | |
| Parkinson’s disease Duration, years | 12.7 (6.3) | ||
| Hoehn & Yahr | “On”Stage | 264 (80) | 2.8 (0.8) |
| “Off” Stage | 253 (77) | 4.0 (0.9) | |
| UPDRS Part II, total score during “on” time | 230 (70) | 16.5 (9.8) | |
| UPDRS Part III, total score during “on” time | 230 (70) | 24.6 (12.0) | |
| UPDRS Part IV, daily hours | Modified Item 39 (“off” time) | 211 (64) | 6.0 (3.2) |
| Modified Item 32 (dyskinesia) | 217 (66) | 4.3 (3.8) | |
| n (% of N=356a) with: | ||||||
| Visit | Oral Levodopab | COMT Inhibitors | MAO-B Inhibitors | DA | Amantadine | Others |
| D1 | 181 (51) | 30 (8.4) | 29 (8.1) | 41 (12) | 25 (7.0) | 31 (8.7) |
| M6 | 147 (41) | 28 (7.9) | 30 (8.4) | 38 (11) | 30 (8.4) | 23 (6.5) |
| M12 | 140 (39) | 25 (7.0) | 28 (7.9) | 45 (13) | 31 (8.7) | 17 (4.8) |
| M18 | 130 (37) | 26 (7.3) | 21 (5.9) | 43 (12) | 27 (7.6) | 17 (4.8) |
| M24 | 117 (33) | 19 (5.3) | 20 (5.6) | 42 (12) | 30 (8.4) | 14 (3.9) |
a. Patients with at least 1 dose of LCIG and 1 post-baseline safety evaluation
b. Includes rescue and night time doses
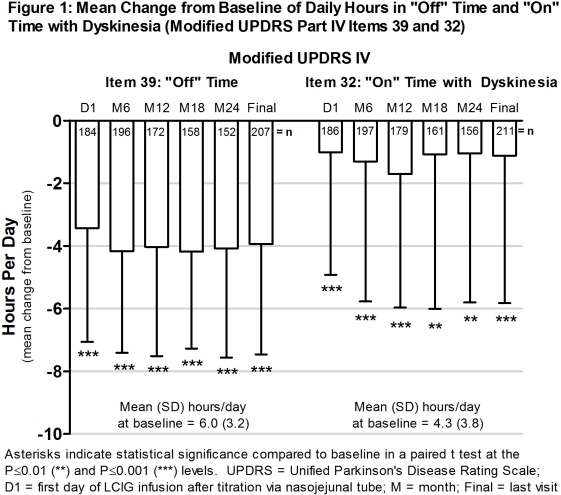
Conclusions: LCIG led to significant improvements in motor fluctuations and dyskinesia in advanced PD patients in this long-term registry. The observed tolerability was consistent with the established safety profile of LCIG. (ref 2). References: 1. Antonini et al. Parkinsonism Relat Disord. 2015; 21(3):231-5. 2. Fernandez et al. Mov Disord. 2015; 30(4):500-9.
To cite this abstract in AMA style:
W. Poewe, T. Kimber, B. Bermans, P. Odin, A. Antonini, O. Bajenaru, K. Onuk, A. Yegin, L. Bergmann, K.R. Chaudhuri. Levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients: Final long-term efficacy results on motor complications from the GLORIA registry [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-in-routine-care-of-advanced-parkinsons-disease-patients-final-long-term-efficacy-results-on-motor-complications-from-the-gloria-registry/. Accessed February 12, 2026.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-in-routine-care-of-advanced-parkinsons-disease-patients-final-long-term-efficacy-results-on-motor-complications-from-the-gloria-registry/
